Home /
Expert Answers /
Chemical Engineering /
by-assuming-that-the-mass-transfer-coefficients-given-in-example-18-3-are-applicable-to-the-proble-pa987
(Solved): By assuming that the mass-transfer coefficients given in Example 18.3 are applicable to the proble ...
By assuming that the mass-transfer coefficients given in Example 18.3 are applicable to the problem described in Example 18.1, proceed to calculate the required tower height for removing 99 % of ammonia by absorption in water. Summarize the results of liquid and gas mass velocities, pressure drop, tower diameter and height for the successful design and operation of this gas absorption tower. (Hint: Example 18.4 can be consulted for equilibrium data) (15%)
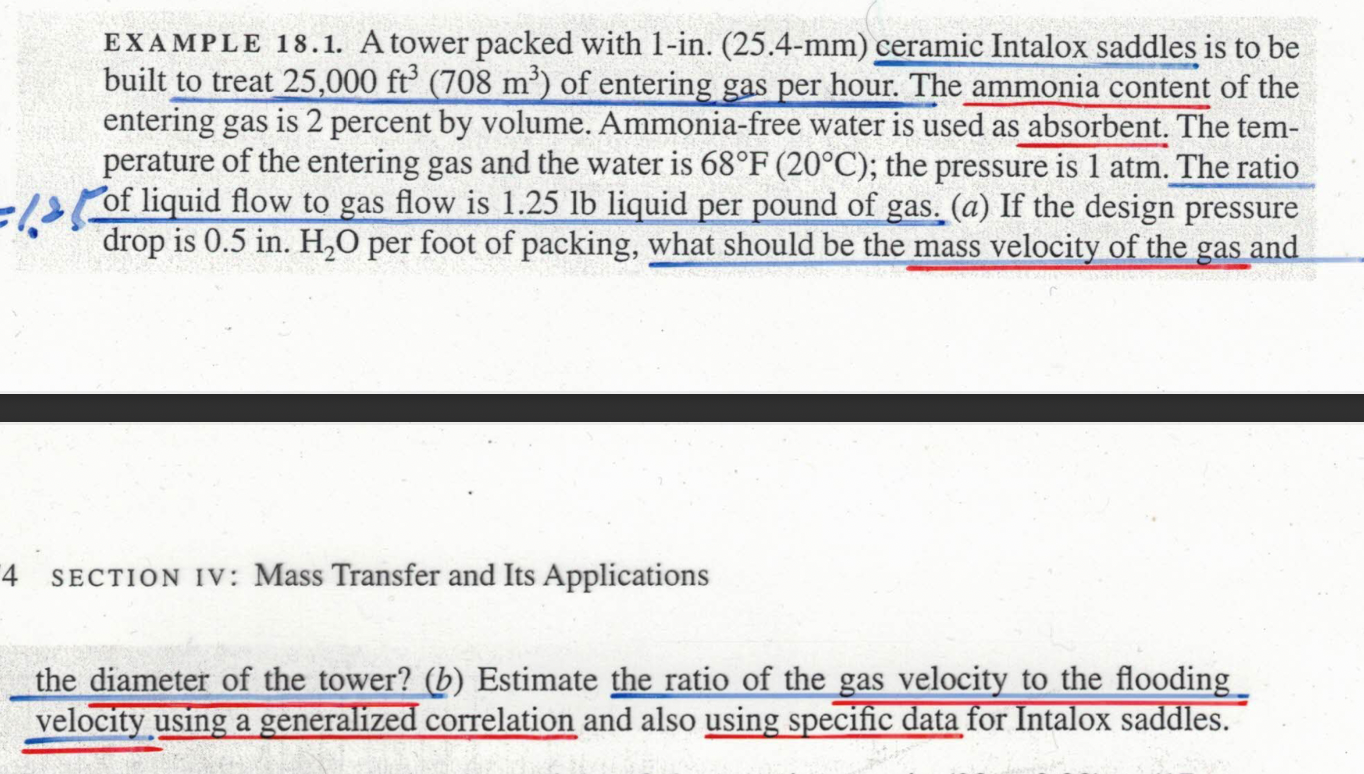
EXAMPLE 18.1. A tower packed with 1-in. (25.4-mm) ceramic Intalox saddles is to be built to treat 25,000 ft³ (708 m³) of entering gas per hour. The ammonia content of the entering gas is 2 percent by volume. Ammonia-free water is used as absorbent. The tem- perature of the entering gas and the water is 68°F (20°C); the pressure is 1 atm. The ratio of liquid flow to gas flow is 1.25 lb liquid per pound of gas. (a) If the design pressure drop is 0.5 in. H?O per foot of packing, what should be the mass velocity of the gas and 4 SECTION IV: Mass Transfer and Its Applications the diameter of the tower? (b) Estimate the ratio of the gas velocity to the flooding velocity using a generalized correlation and also using specific data for Intalox saddles.
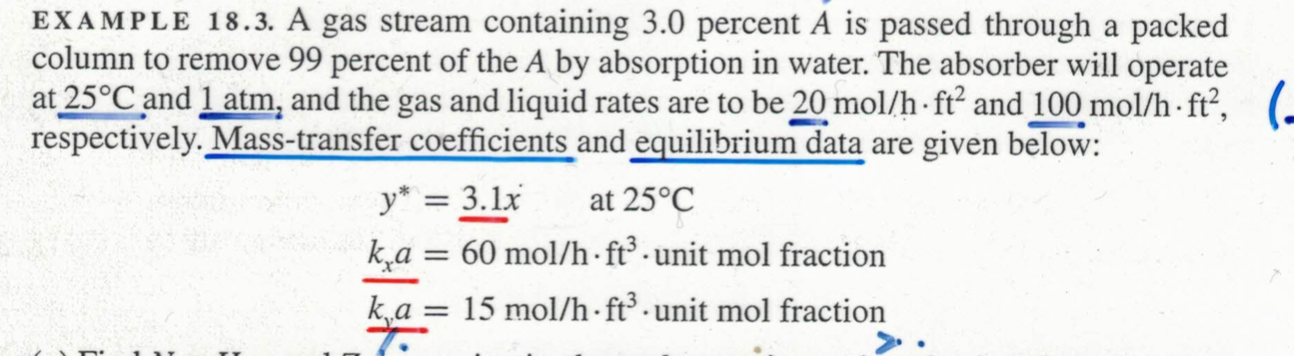
EXAMPLE 18.3. A gas stream containing 3.0 percent A is passed through a packed column to remove 99 percent of the A by absorption in water. The absorber will operate at 25°C and 1 atm, and the gas and liquid rates are to be 20 mol/h ft² and 100 mol/h ft², respectively. Mass-transfer coefficients and equilibrium data are given below: y* = 3.1x at 25°C ka = 60 mol/h ft³ unit mol fraction ka = 15 mol/h ft³ unit mol fraction
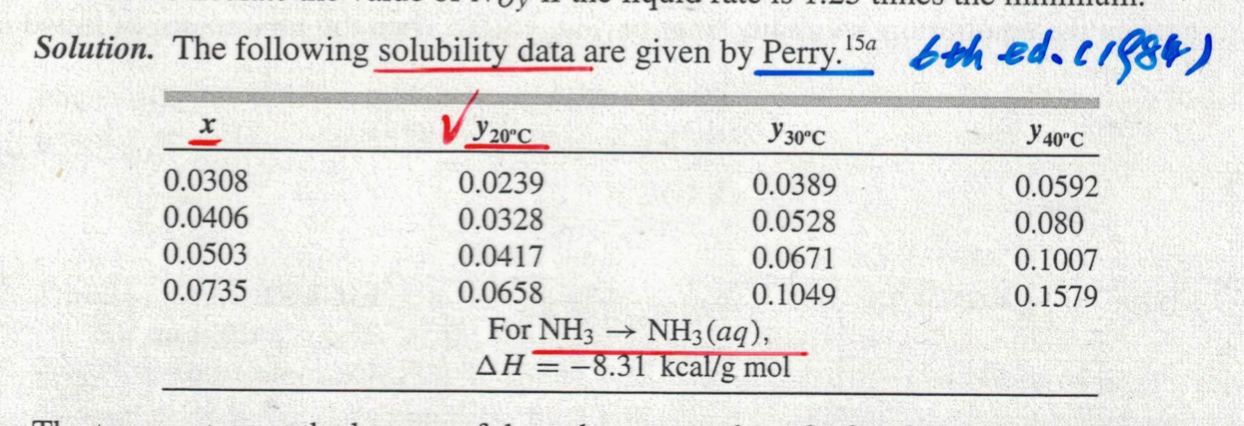
Solution. The following solubility data are given by Perry. 15a 6th ed. 1984) Y20°C Y 30°C Y 40°C 0.0308 0.0239 0.0389 0.0592 0.0406 0.0328 0.0528 0.080 0.0503 0.0417 0.0671 0.1007 0.0735 0.0658 0.1049 0.1579 For NH3 ? NH3(aq), AH = -8.31 kcal/g mol
Expert Answer
Solution: Given that, A tower packed with 1-in. (25.4-mm) ceramic Intalox saddles is to be built to treat 25,000 ft3 (708 m³) of entering gas per hour. The ammonia content of the entering gas is 2 percent by volume. Ammonia-free water is used as abso