Home /
Expert Answers /
Chemical Engineering /
c-rutile-the-high-temperature-form-of-titanium-oxide-tio2-adopts-a-tetragonal-p-structure-with-pa880
(Solved): c) Rutile, the high-temperature form of titanium oxide, TiO2, adopts a tetragonal P structure with ...
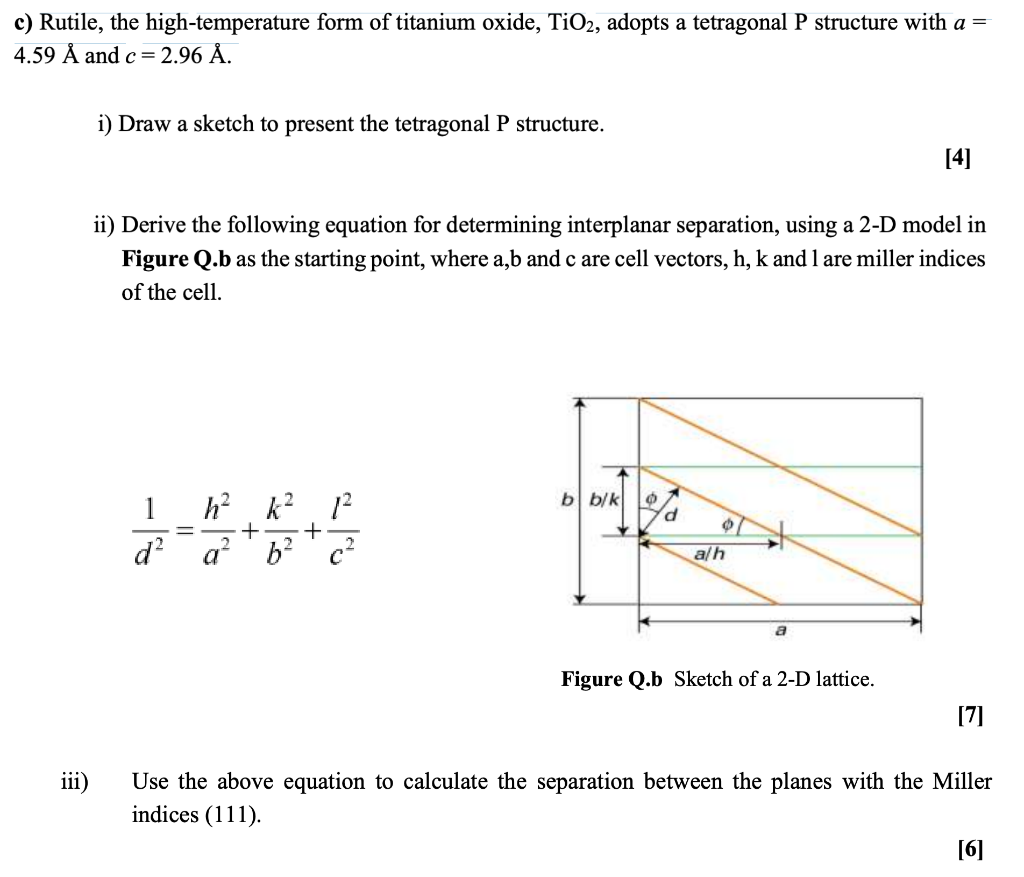
c) Rutile, the high-temperature form of titanium oxide, TiO2, adopts a tetragonal P structure with a = 4.59 Å and c = 2.96 Å. i) Draw a sketch to present the tetragonal P structure. [4] ii) Derive the following equation for determining interplanar separation, using a 2-D model in Figure Q.b as the starting point, where a,b and c are cell vectors, h, k and 1 are miller indices of the cell. b b/k h² 1² o a/h a Figure Q.b Sketch of a 2-D lattice. [7] iii) Use the above equation to calculate the separation between the planes with the Miller indices (111). [6] + 16 + d