Home /
Expert Answers /
Chemistry /
calculate-the-freezing-point-of-a-solution-prepared-by-dissolving-110-mathrm-g-of-sucrose-pa170
(Solved): Calculate the freezing point of a solution prepared by dissolving \( 110 \mathrm{~g} \) of sucrose, ...
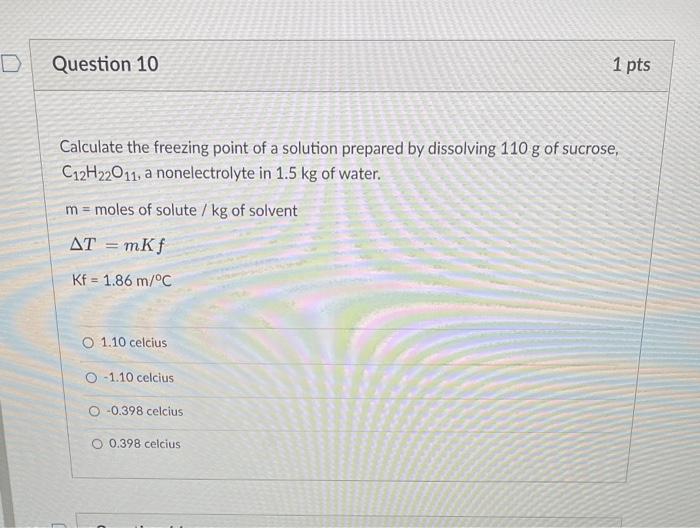
Calculate the freezing point of a solution prepared by dissolving \( 110 \mathrm{~g} \) of sucrose, \( \mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11} \), a nonelectrolyte in \( 1.5 \mathrm{~kg} \) of water. \( m= \) moles of solute \( / \mathrm{kg} \) of solvent \[ \begin{array}{l} \Delta T=m K f \\ \mathrm{Kf}=1.86 \mathrm{~m} /{ }^{\circ} \mathrm{C} \end{array} \] \( 1.10 \) celcius \( -1.10 \) celcius \( -0.398 \) celcius \( 0.398 \) celcius