Home /
Expert Answers /
Chemistry /
calculate-the-volume-in-liters-of-a-1-69-times-10-4-mu-m-magnesium-fluoride-solution-that-contain-pa588
(Solved): Calculate the volume in liters of a 1.69\times 10^(-4)\mu M magnesium fluoride solution that contain ...
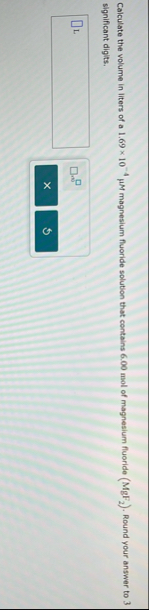
Calculate the volume in liters of a
1.69\times 10^(-4)\mu Mmagnesium fluoride solution that contains 6.00 mol of magnesium fluoride (
MgF_(2)). Round your answer to 3 significant digits.