Home /
Expert Answers /
Chemical Engineering /
combustion-of-pulverized-carbon-particles-of-70-mu-m-in-diameter-is-taking-place-in-pure-o-2-at-12-pa335
(Solved): Combustion of pulverized carbon particles of 70\mu m in diameter is taking place in pure O_(2) at 12 ...
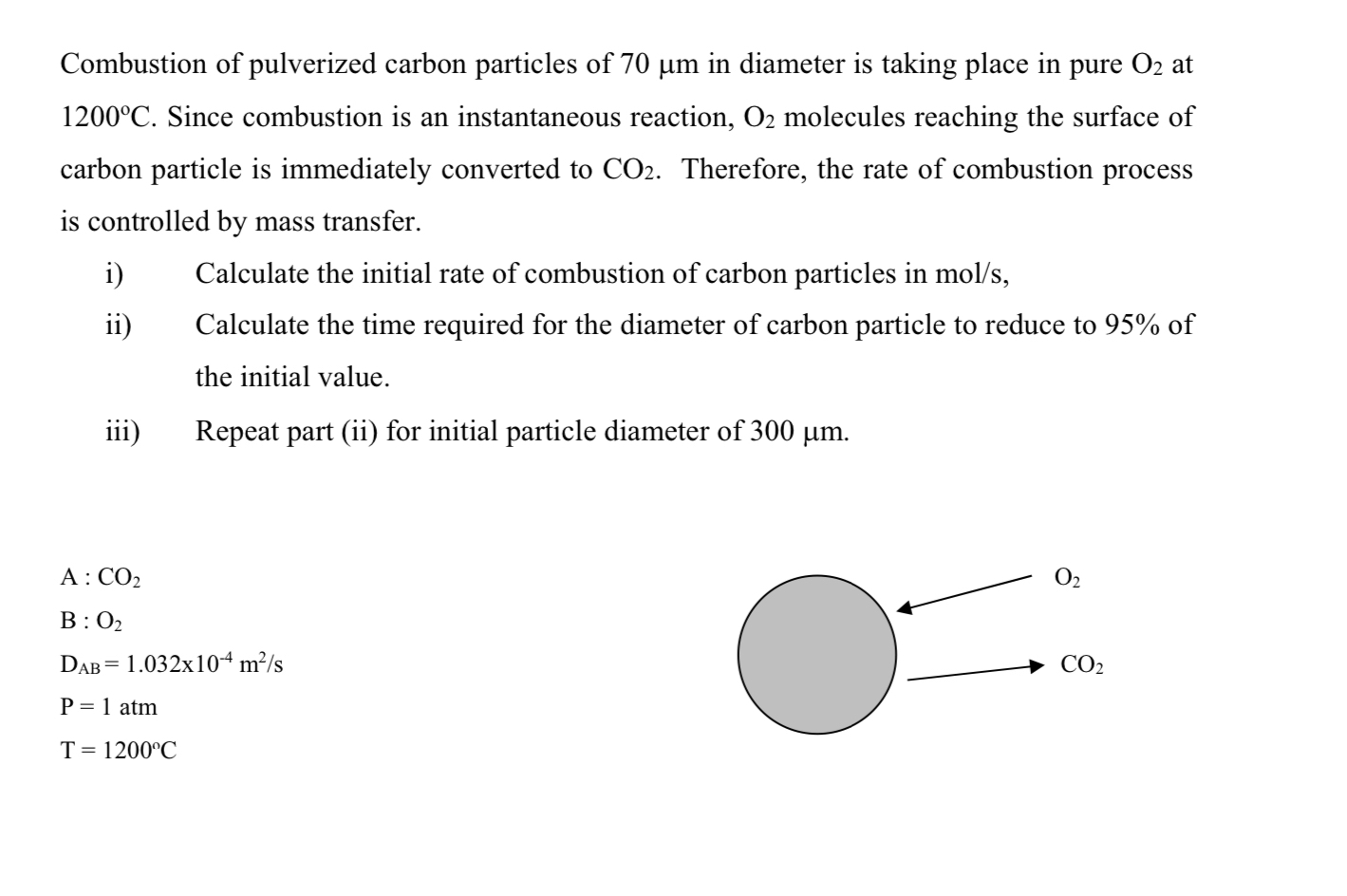
Combustion of pulverized carbon particles of
70\mu min diameter is taking place in pure
O_(2)at
1200\deg C. Since combustion is an instantaneous reaction,
O_(2)molecules reaching the surface of carbon particle is immediately converted to
CO_(2). Therefore, the rate of combustion process is controlled by mass transfer. i) Calculate the initial rate of combustion of carbon particles in
mo(l)/(s), ii) Calculate the time required for the diameter of carbon particle to reduce to
95%of the initial value. iii) Repeat part (ii) for initial particle diameter of
300\mu m. A:
CO_(2)B :
O_(2)
D_(AB)=1.032\times 10^(-4)(m^(2))/(s)
P=1atm
T=1200\deg C