Home /
Expert Answers /
Chemistry /
complete-the-following-chemical-reactions-and-balance-them-if-no-reaction-write-no-reaction-remem-pa265
(Solved): Complete the following chemical reactions and balance them. If NO REACTION write no reaction. Remem ...
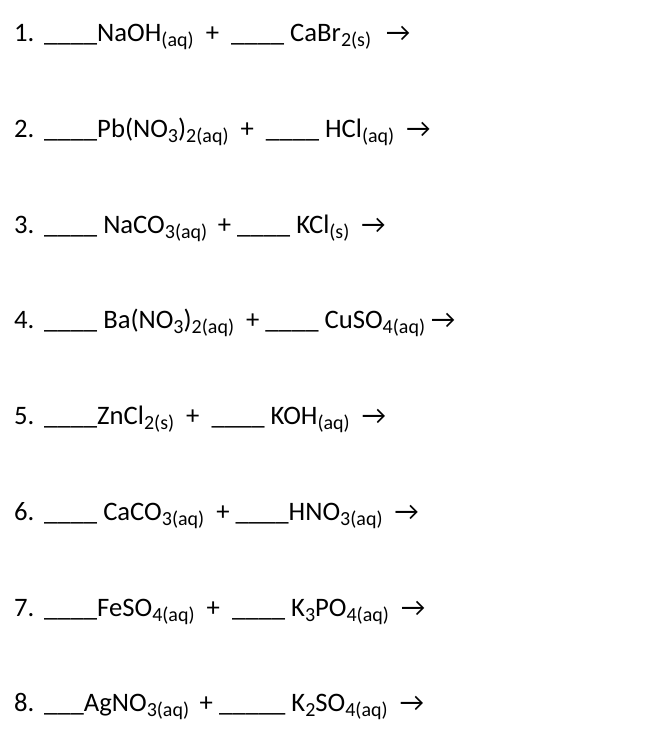
Complete the following chemical reactions and balance them. If NO REACTION write no reaction. Remember the states. Each equation is out of 3 marks.
if both products are soluble, then there is no reaction.
\( \mathrm{NaOH}_{(\mathrm{aq})}+\mathrm{CaBr}_{2(\mathrm{~s})} \rightarrow \) \( \mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2(\mathrm{aq})}+\mathrm{HCl}_{(\mathrm{aq})} \rightarrow \) \( \mathrm{NaCO}_{3(\mathrm{aq})}+\mathrm{K}^{+} \mathrm{KCl}_{(\mathrm{s})} \rightarrow \) \( \mathrm{Ba}\left(\mathrm{NO}_{3}\right)_{2(\mathrm{aq})}+\mathrm{CuSO}_{4(\mathrm{aq})} \rightarrow \) \( \mathrm{ZnCl}_{2(\mathrm{~s})}+\mathrm{KOH}_{(\mathrm{aq})} \rightarrow \) \( \mathrm{CaCO}_{3(\mathrm{aq})}+\mathrm{HNO}_{3(\mathrm{aq})} \rightarrow \) \( \operatorname{CeSO}_{4(\mathrm{aq})}+\mathrm{Fe}_{3} \mathrm{PO}_{4(\mathrm{aq})} \rightarrow \) \( \mathrm{AgNO}_{3(\mathrm{aq})}+\mathrm{K}_{2} \mathrm{SO}_{4(\mathrm{aq})} \rightarrow \)
Expert Answer
Answer: 1. When sodium hydroxide reacts with the calcium bromide it result in the formation of calcium hydroxide and sodium bromide. The complete balanced chemical reaction is as follows below. 2NaOH + CaBr2 2NaBr + Ca(OH)2 2. When lead nitrate is cr