Home /
Expert Answers /
Chemistry /
complete-the-right-side-of-the-following-molecular-equations-then-write-the-net-ionic-equations-as-pa795
(Solved): Complete the right side of the following molecular equations. Then write the net ionic equations. As ...
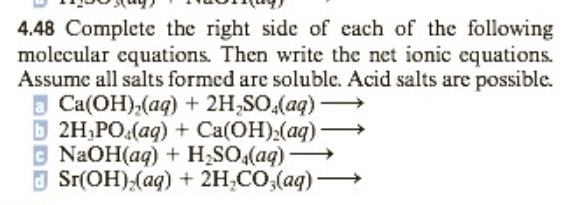
Complete the right side of the following molecular equations. Then write the net ionic equations. Assume all salts formed are soluble. Acid salts are possible:
a.) Ca(OH)2(aq) + 2H2SO4(aq)->
b.) 2H3PO4(aq) + Ca(OH)2(aq)->
c.) NaOH(aq) + H2SO4(aq)->
d.) Sr(OH)2(aq) + 2H2CO3(aq)->
4.48 Complete the right side of each of the following molecular equations. Then write the net ionic equations. Assume all salts formed are soluble. Acid salts are possible.
Expert Answer
To complete the right side of the molecular equati...