Home /
Expert Answers /
Chemistry /
compound-a-exhibits-a-peak-in-its-1h-nmr-spectrum-at-7-6ppm-indicating-that-it-is-aromatic-in-or-pa888
(Solved): Compound A exhibits a peak in its 1H NMR spectrum at 7.6ppm, indicating that it is aromatic. In or ...
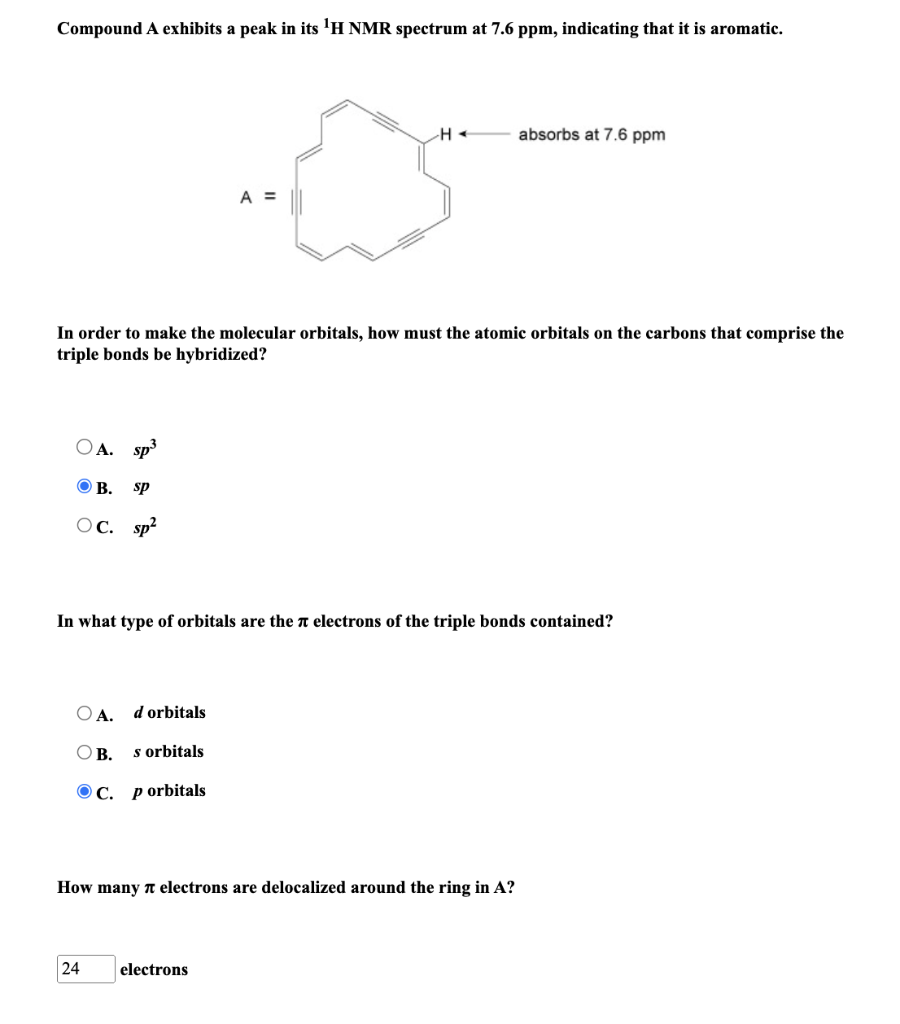
Compound A exhibits a peak in its NMR spectrum at , indicating that it is aromatic. In order to make the molecular orbitals, how must the atomic orbitals on the carbons that comprise the triple bonds be hybridized? A. B. C. In what type of orbitals are the electrons of the triple bonds contained? A. orbitals B. orbitals C. p orbitals How many electrons are delocalized around the ring in ? electrons