Home /
Expert Answers /
Chemistry /
consider-k-and-delta-g-deg-for-the-following-reactions-note-reference-the-fundamental-constants-pa501
(Solved): Consider K and \Delta G\deg for the following reactions. Note: Reference the Fundamental constants ...
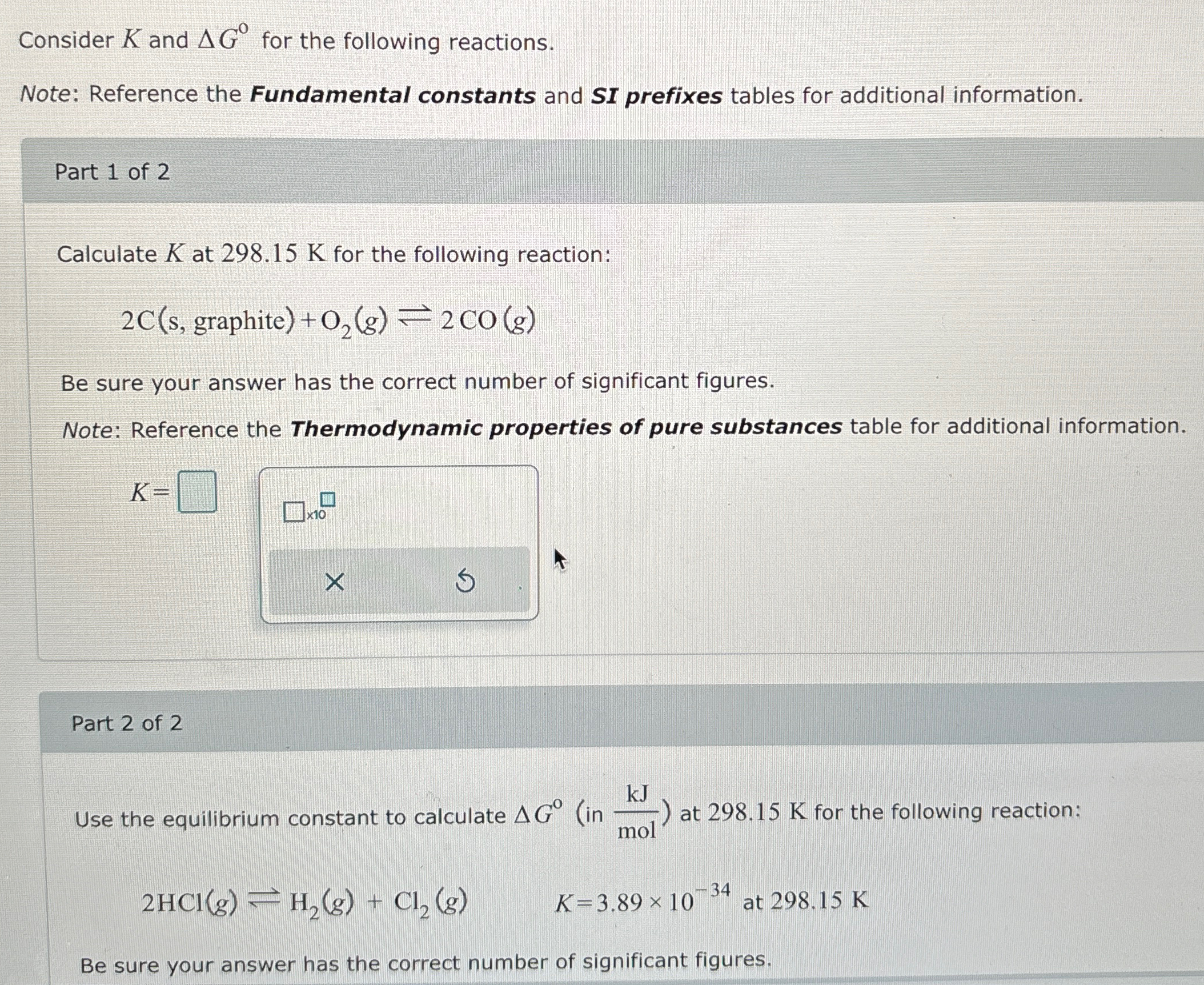
Consider
Kand
\Delta G\deg for the following reactions. Note: Reference the Fundamental constants and SI prefixes tables for additional information. Part 1 of 2 Calculate
Kat
298.15Kfor the following reaction:
2C(s, graphite )+O_(2)(g)⇌2CO(g)Be sure your answer has the correct number of significant figures. Note: Reference the Thermodynamic properties of pure substances table for additional information.
K=Part 2 of 2 Use the equilibrium constant to calculate
\Delta G\deg (in
(kJ)/(mol)) at
298.15Kfor the following reaction:
2HCl(g)⇌H_(2)(g)+Cl_(2)(g),K=3.89\times 10^(-34) at 298.15KBe sure your answer has the correct number of significant figures.