Home /
Expert Answers /
Chemistry /
consider-the-equilibrium-system-described-by-the-chemical-reaction-below-at-equilibrium-a-1-5-pa494
(Solved): Consider the equilibrium system described by the chemical reaction below. At equilibrium, a \( 1.5 ...
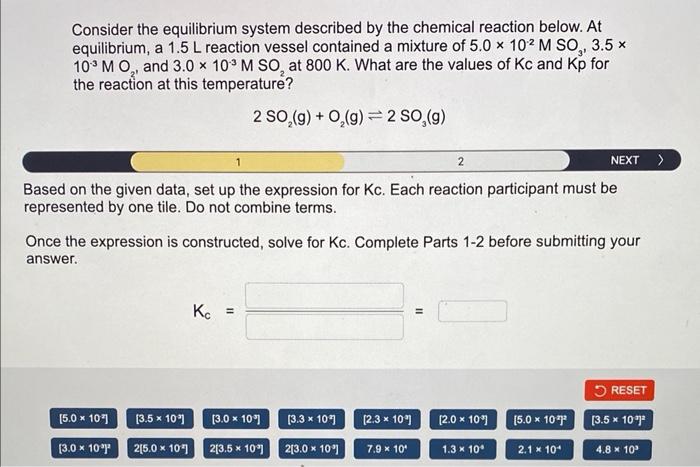
Consider the equilibrium system described by the chemical reaction below. At equilibrium, a \( 1.5 \mathrm{~L} \) reaction vessel contained a mixture of \( 5.0 \times 10^{-2} \mathrm{M} \mathrm{SO}_{3}, 3.5 \times \) \( 10^{-3} \mathrm{MO}_{2} \), and \( 3.0 \times 10^{-3} \mathrm{M} \mathrm{SO}_{2} \) at \( 800 \mathrm{~K} \). What are the values of \( \mathrm{Kc} \) and \( \mathrm{Kp} \) for the reaction at this temperature? \[ 2 \mathrm{SO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{SO}_{3}(\mathrm{~g}) \] Based on the given data, set up the expression for Kc. Each reaction participant must be epresented by one tile. Do not combine terms. Once the expression is constructed, solve for \( \mathrm{Kc} \). Complete Parts 1-2 before submitting your answer.
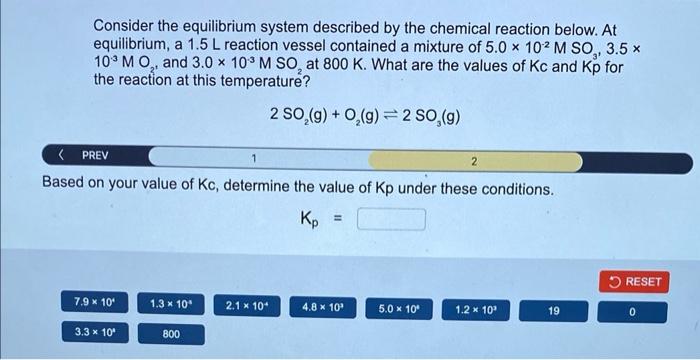
Consider the equilibrium system described by the chemical reaction below. At equilibrium, a \( 1.5 \mathrm{~L} \) reaction vessel contained a mixture of \( 5.0 \times 10^{-2} \mathrm{M} \mathrm{SO}_{3}, 3.5 \times \) \( 10^{-3} \mathrm{MO}_{2^{\text {an }}} \) and \( 3.0 \times 10^{-3} \mathrm{M} \mathrm{SO}_{2} \) at \( 800 \mathrm{~K} \). What are the values of \( \mathrm{Kc} \) and \( \mathrm{Kp} \) for the reaction at this temperature? \[ 2 \mathrm{SO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{SO}_{3}(\mathrm{~g}) \] < PREV Based on your value of \( \mathrm{Kc} \), determine the value of \( \mathrm{Kp} \) under these conditions. \[ K_{p}= \]