Home /
Expert Answers /
Chemistry /
consider-the-following-bronsted-acid-base-reaction-at-25-deg-c-hcl-aq-f-aq-hf-aq-cl-pa962
(Solved): Consider the following Bronsted acid-base reaction at 25\deg C : HCl(aq)+F^(-)(aq)HF(aq)+Cl^(-)( ...
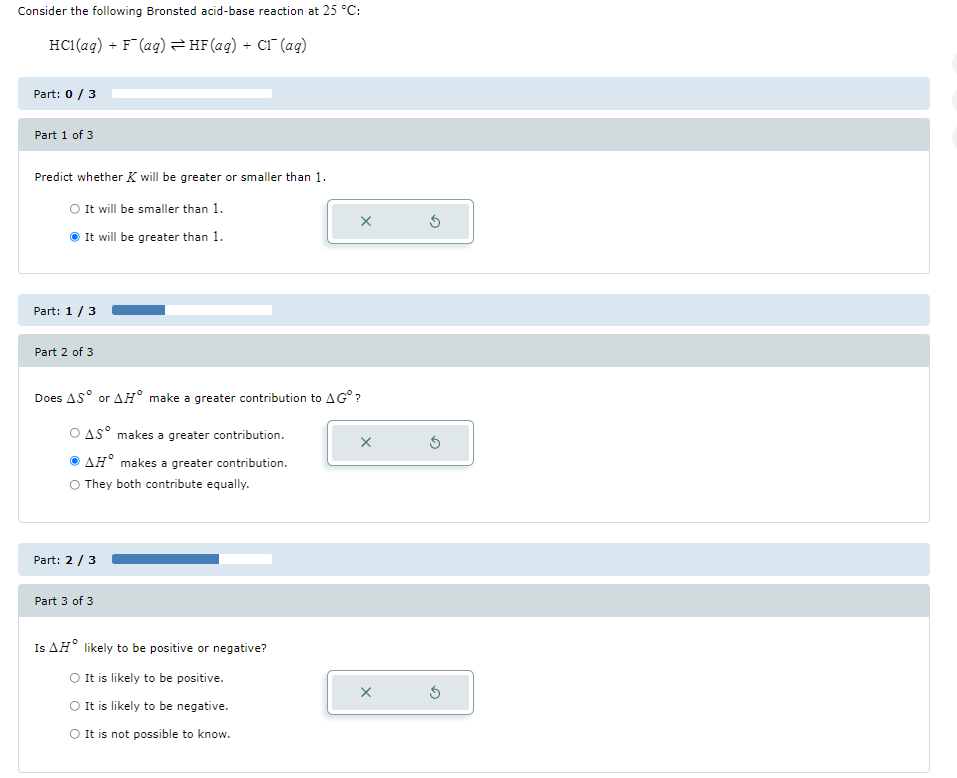
Consider the following Bronsted acid-base reaction at
25\deg C:
HCl(aq)+F^(-)(aq)⇌HF(aq)+Cl^(-)(aq)Part:
(0)/(3)Part 1 of 3 Predict whether
Kwill be greater or smaller than 1 . It will be smaller than 1 . It will be greater than 1 . Part:
(1)/(3)Part 2 of 3 Does
\Delta S\deg or
\Delta H\deg make a greater contribution to
\Delta G\deg ?
\Delta S\deg makes a greater contribution.
\Delta H\deg makes a greater contribution. They both contribute equally. Part:
(2)/(3)Part 3 of 3 Is
\Delta H\deg likely to be positive or negative? It is likely to be positive. It is likely to be negative. It is not possible to know.