Home /
Expert Answers /
Chemistry /
consider-the-partial-reaction-shown-below-a-draw-the-structure-of-a-starting-material-that-can-r-pa243
(Solved): Consider the partial reaction shown below. a. Draw the structure of a starting material that can r ...
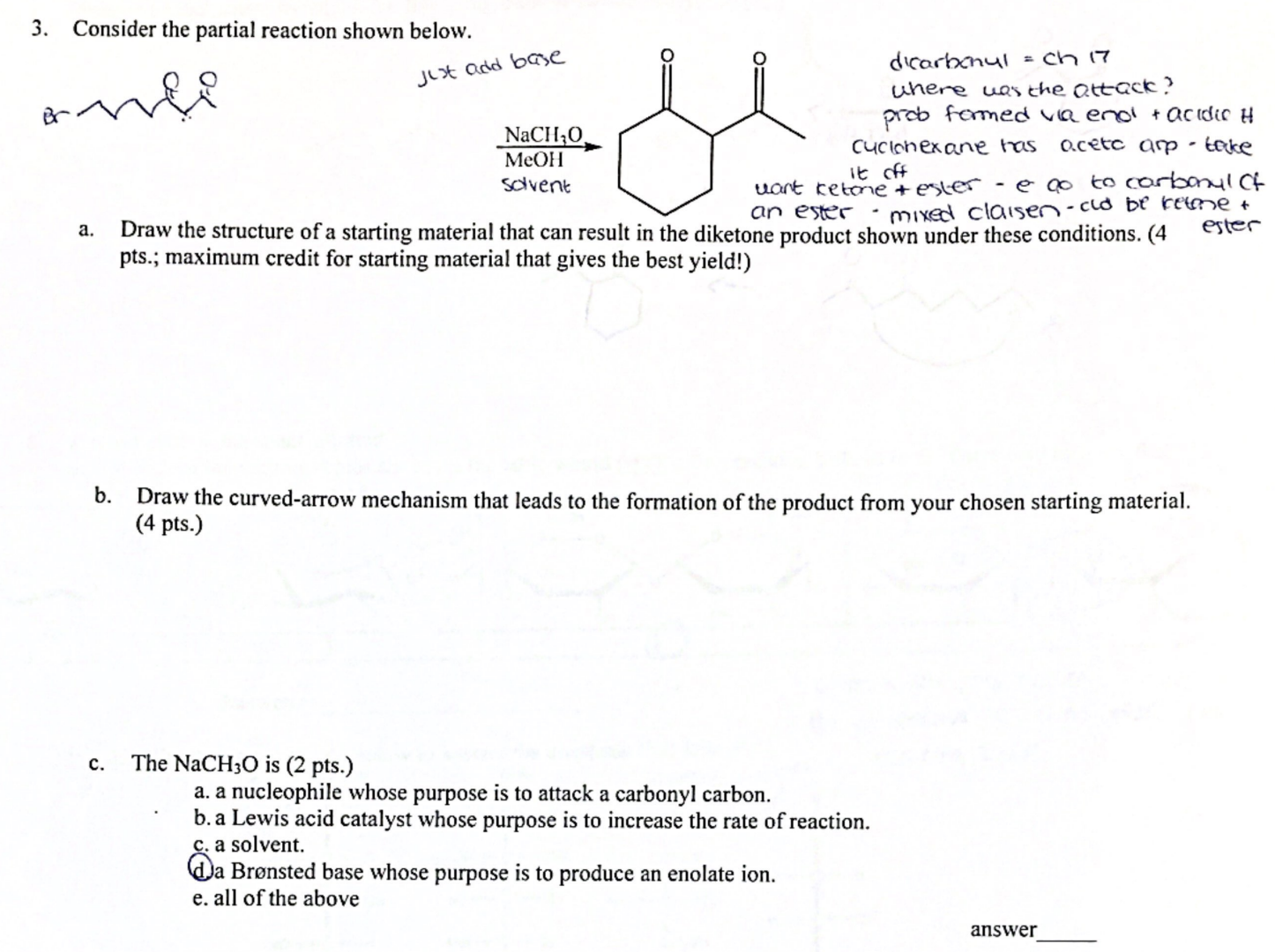
Consider the partial reaction shown below. a. Draw the structure of a starting material that can result in the diketone product shown mixed claisen-clo be retone + - cose conditions. (4 ester pts.; maximum credit for starting material that gives the best yield!) b. Draw the curved-arrow mechanism that leads to the formation of the product from your chosen starting material. (4 pts.) c. The
NaCH_(3)Ois (2 pts.) a. a nucleophile whose purpose is to attack a carbonyl carbon. b. a Lewis acid catalyst whose purpose is to increase the rate of reaction. c. a solvent. d. Brønsted base whose purpose is to produce an enolate ion. e. all of the above