Home /
Expert Answers /
Chemistry /
consider-the-particle-in-a-1d-box-whose-wave-function-is-given-by-x-l2-pa246
(Solved): Consider the particle in a 1D-box, whose wave function is given by: (x)=(L2 ...
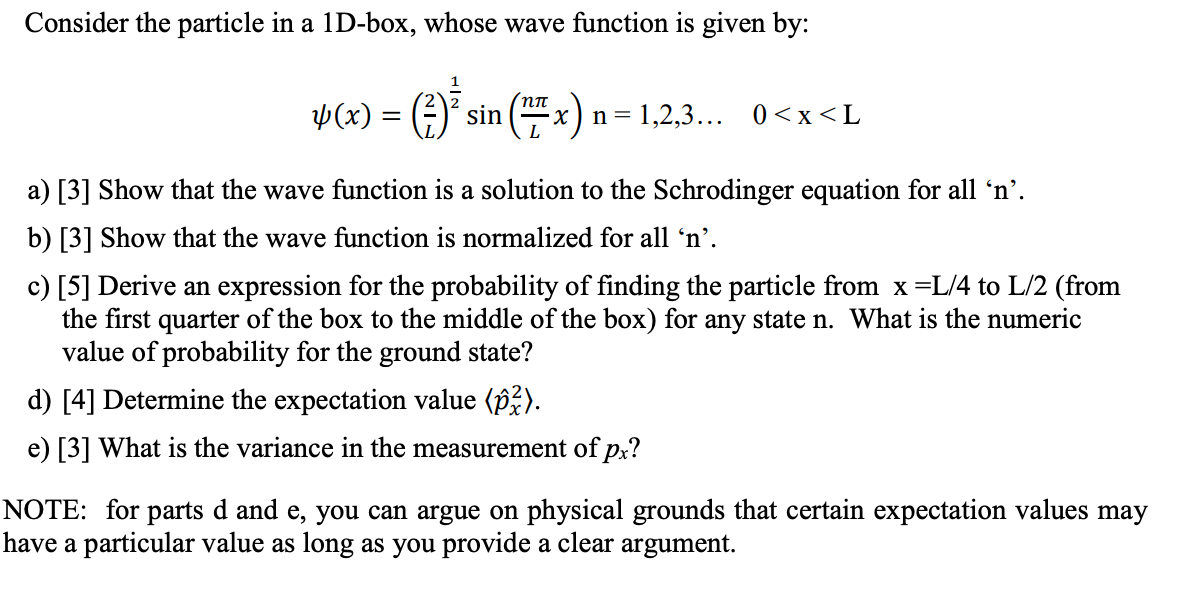
Consider the particle in a 1D-box, whose wave function is given by: a) [3] Show that the wave function is a solution to the Schrodinger equation for all ' '. b) [3] Show that the wave function is normalized for all ' '. c) [5] Derive an expression for the probability of finding the particle from to (from the first quarter of the box to the middle of the box) for any state . What is the numeric value of probability for the ground state? d) [4] Determine the expectation value . e) [3] What is the variance in the measurement of ? NOTE: for parts d and e, you can argue on physical grounds that certain expectation values may have a particular value as long as you provide a clear argument.
Expert Answer
a) To show that the wave function is a solution to the Schrödinger equation for all 'n', we will substitute the wave function into the time-independen