Home /
Expert Answers /
Chemical Engineering /
consider-the-reaction-ch3oh-l-ch4-g-1-2o2-g-part-a-calculate-rh29s-express-pa133
(Solved): Consider the reaction CH3OH(l)CH4(g)+1/2O2(g) Part A Calculate rH29s Express ...
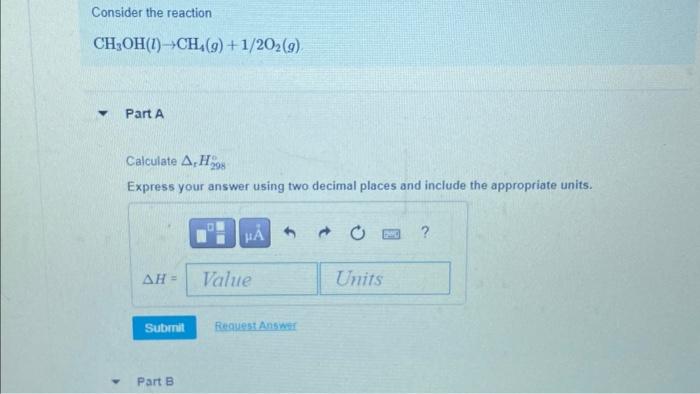
Consider the reaction Part A Calculate Express your answer using two decimal places and include the appropriate units.
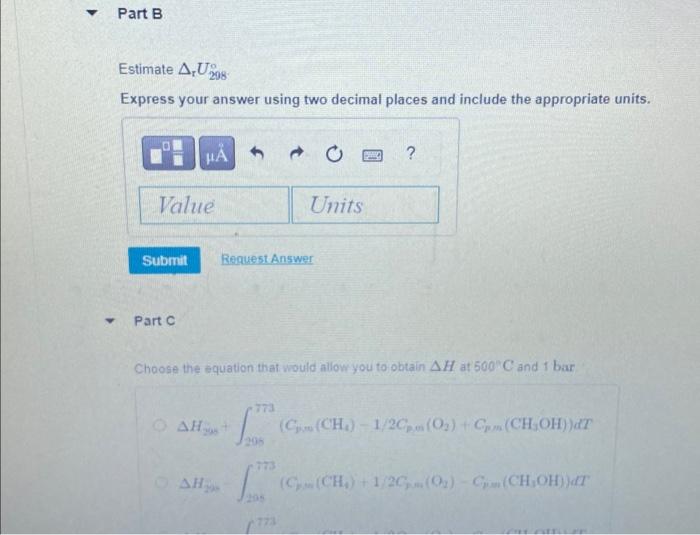
Estimate Express your answer using two decimal places and include the appropriate units. Part C Choose the equation that would allow you to obtain at and 1 bar
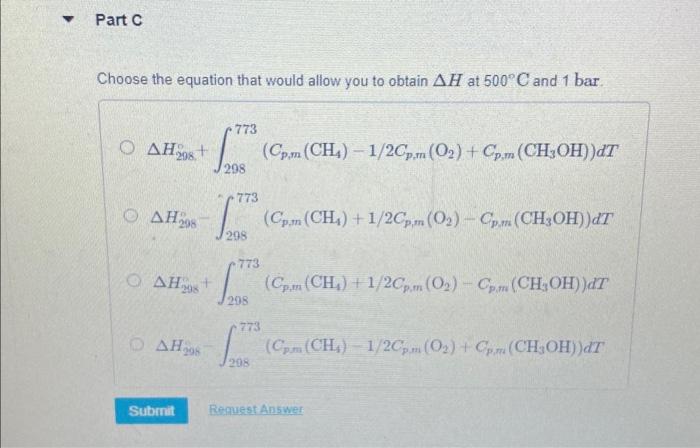
Choose the equation that would allow you to obtain at and .