Home /
Expert Answers /
Chemistry /
consider-the-reversible-carnot-cycle-shown-in-the-figure-below-with-1-90mol-of-an-ideal-gas-with-pa740
(Solved): Consider the reversible Carnot cycle shown in the figure below with 1.90mol of an ideal gas with ...
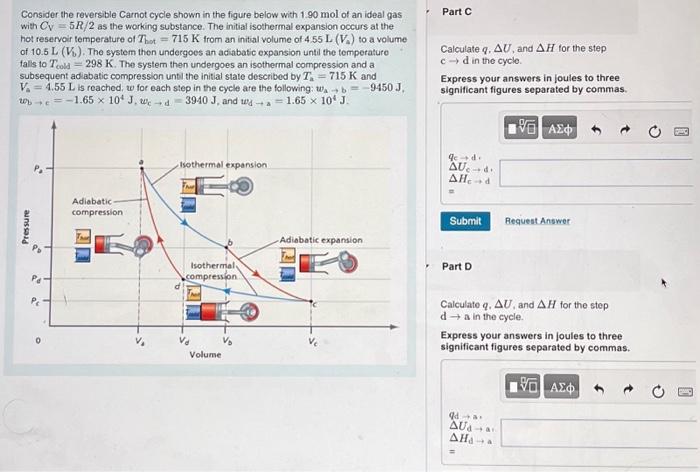
Consider the reversible Carnot cycle shown in the figure below with of an ideal gas with as the working substance. The initial isothermal expansion occurs at the hot reservoif temperature of from an initial volume of to a volume of . The system then undergoes an adiabatic expansion until the temperature falls to . The system then undergoes an isothermal compression and a subsequent adiabatic compression unti the initial state described by and is reached, for each step in the cycle are the following: , , and Part C Calculate , and tor the step in the cycle. Express your answers in joules to three significant figures separated by commas. Part D Calculate , and for the step in the cycle. Express your answers in joules to three significant figures separated by commas.