Home /
Expert Answers /
Chemistry /
dinitrogen-tetraoxide-is-a-colorless-oas-that-dissociates-into-nitrogen-dioxide-a-reddish-brown-gas-pa441
(Solved): Dinitrogen tetraoxide is a colorless oas that dissociates into nitrogen dioxide, a reddish brown gas ...
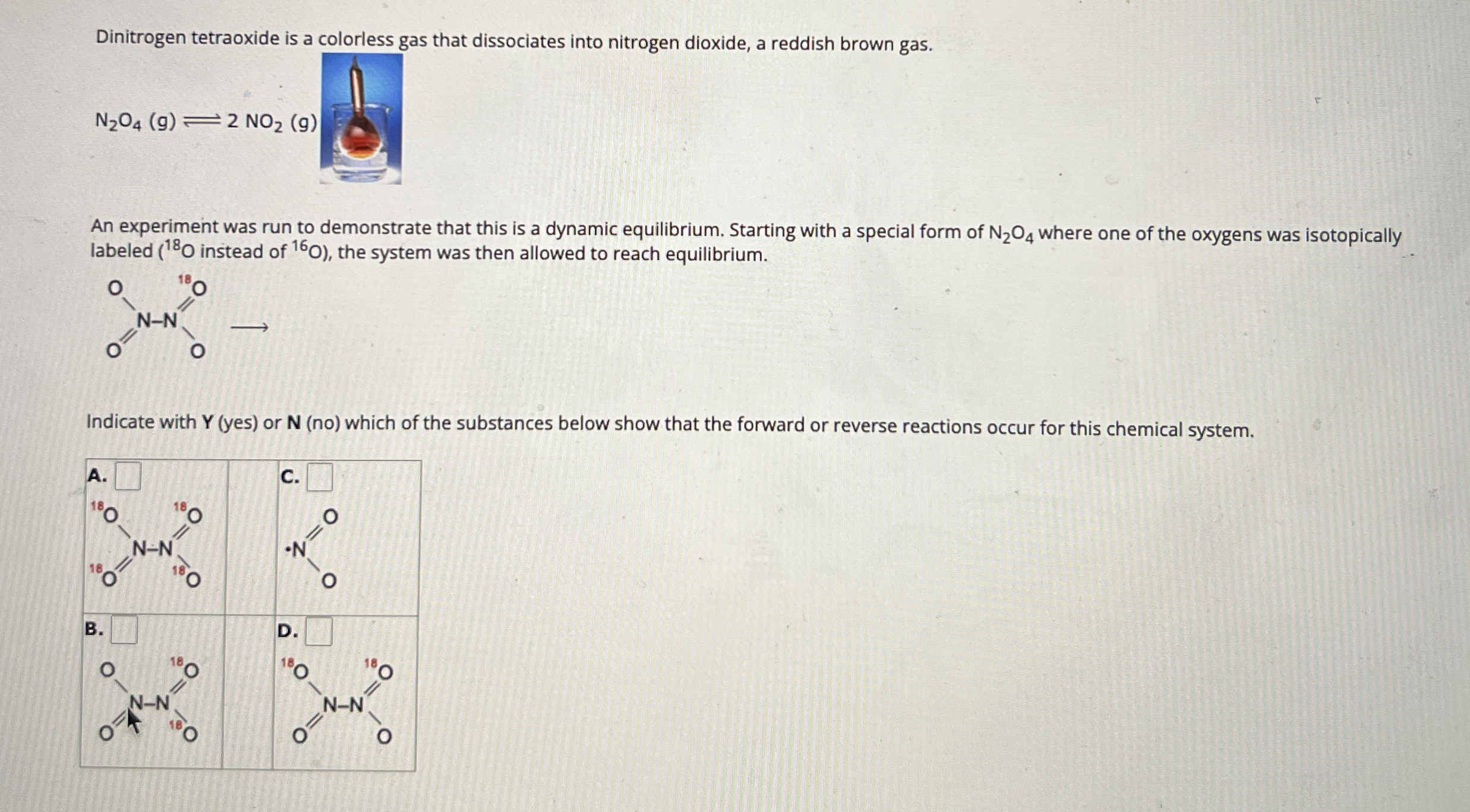
Dinitrogen tetraoxide is a colorless oas that dissociates into nitrogen dioxide, a reddish brown gas. An experiment was run to demonstrate that this is a dynamic equilibrium. Starting with a special form of
N_(2)O_(4)where one of the oxygens was isotopically labeled (
^(18)Oinstead of
^(16)O), the system was then allowed to reach equilibrium. Indicate with
Y(yes)or
N(no)which of the substances below show that the forward or reverse reactions occur for this chemical system.