Home /
Expert Answers /
Chemistry /
do-not-forget-the-proper-coefficient-aq-1m-s-g-etcper-chegg-guidlines-a-maximum-of-4-questions-pa243
(Solved): DO NOT FORGET the proper coefficient: aq, 1M (s) (g) etcper chegg guidlines a maximum of 4 questions ...
DO NOT FORGET the proper coefficient: aq, 1M (s) (g) etc
per chegg guidlines a maximum of 4 questions can be asked. so PLEASE ANSWER EVERY question listed.
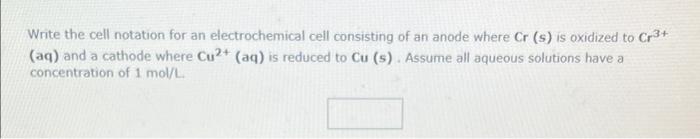
Write the cell notation for an electrochemical cell consisting of an anode where \( \mathrm{Cr} \) (s) is oxidized to \( \mathrm{Cr}^{3+} \) (aq) and a cathode where \( \mathrm{Cu}^{2+}(\mathrm{aq}) \) is reduced to \( \mathrm{Cu} \) (s). Assume all aqueous solutions have a concentration of \( 1 \mathrm{~mol} / \mathrm{L} \).
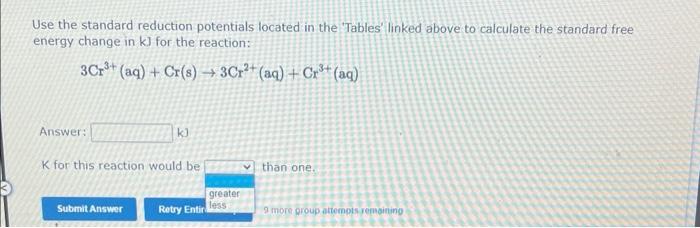
Use the standard reduction potentials located in the 'Tables' linked above to calculate the standard free energy change in \( k] \) for the reaction: \[ 3 \mathrm{Cr}^{3+}(\mathrm{aq})+\mathrm{Cr}(\mathrm{s}) \rightarrow 3 \mathrm{Cr}^{2+}(\mathrm{aq})+\mathrm{Cr}^{3+}(\mathrm{aq}) \]