Home /
Expert Answers /
Chemistry /
draw-bond-hine-structures-for-each-compound-8-pts-use-curved-arrows-to-show-a-resonance-structure-pa854
(Solved): Draw bond-Hine structures for each compound: (8 pts) Use curved arrows to show a resonance structure ...
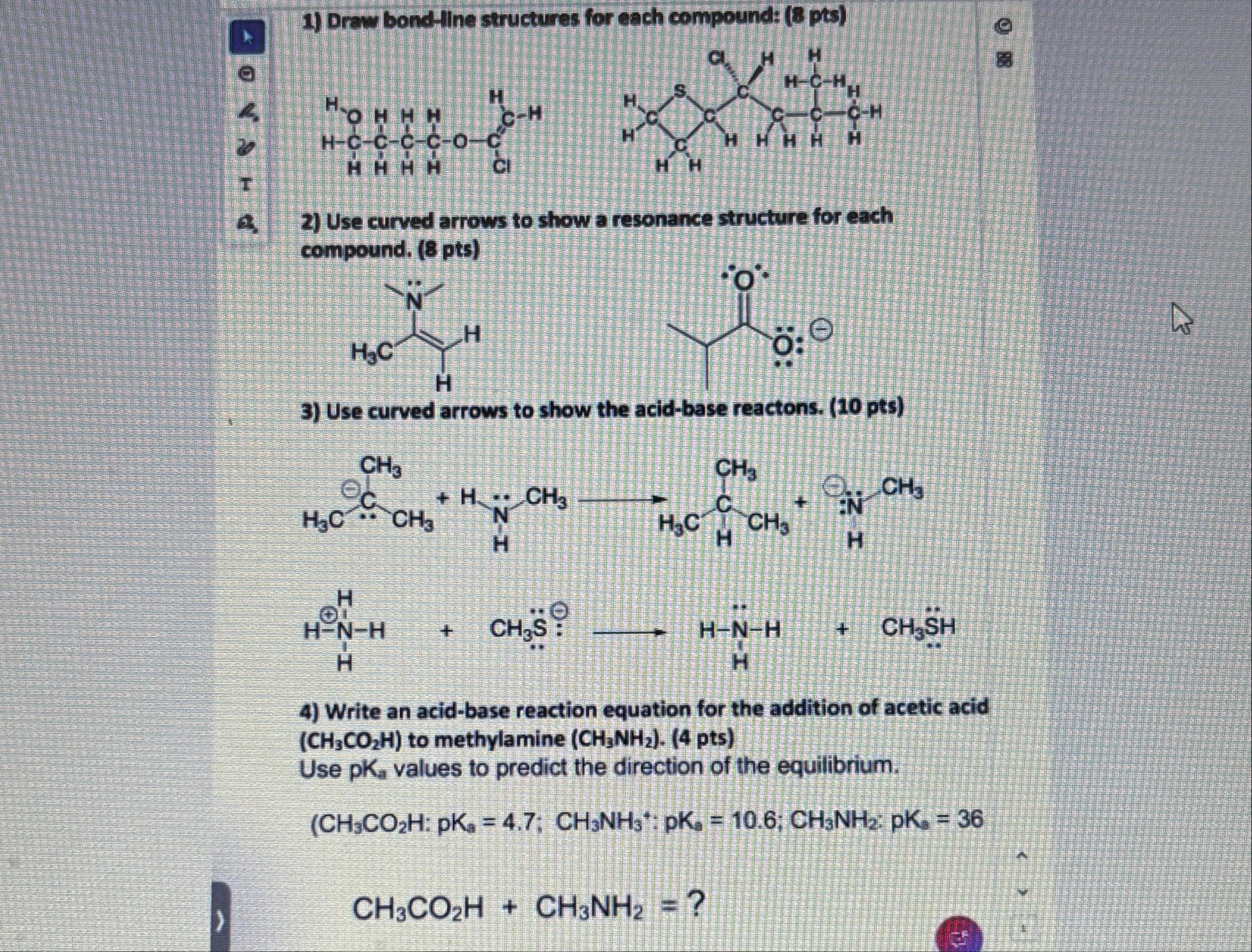
Draw bond-Hine structures for each compound: (8 pts) Use curved arrows to show a resonance structure for each compound. (8 pts) Use curved arrows to show the acid-base reactons. (10 pts) Write an acid-base reaction equation for the addition of acetic acid (
CH_(3)CO_(2)H) to methylamine (
CH_(3)NH_(2)). (4 pts) Use
pK_(a)values to predict the direction of the equilibrium.
CH_(3)CO_(2)H CH_(3)NH_(2)= ?