Home /
Expert Answers /
Chemistry /
draw-the-resonance-structure-of-the-enolate-ion-draw-a-lewis-structure-for-mathrm-so-2-in-pa295
(Solved): Draw the resonance structure of the enolate ion. Draw a Lewis structure for \( \mathrm{SO}_{2} \) in ...
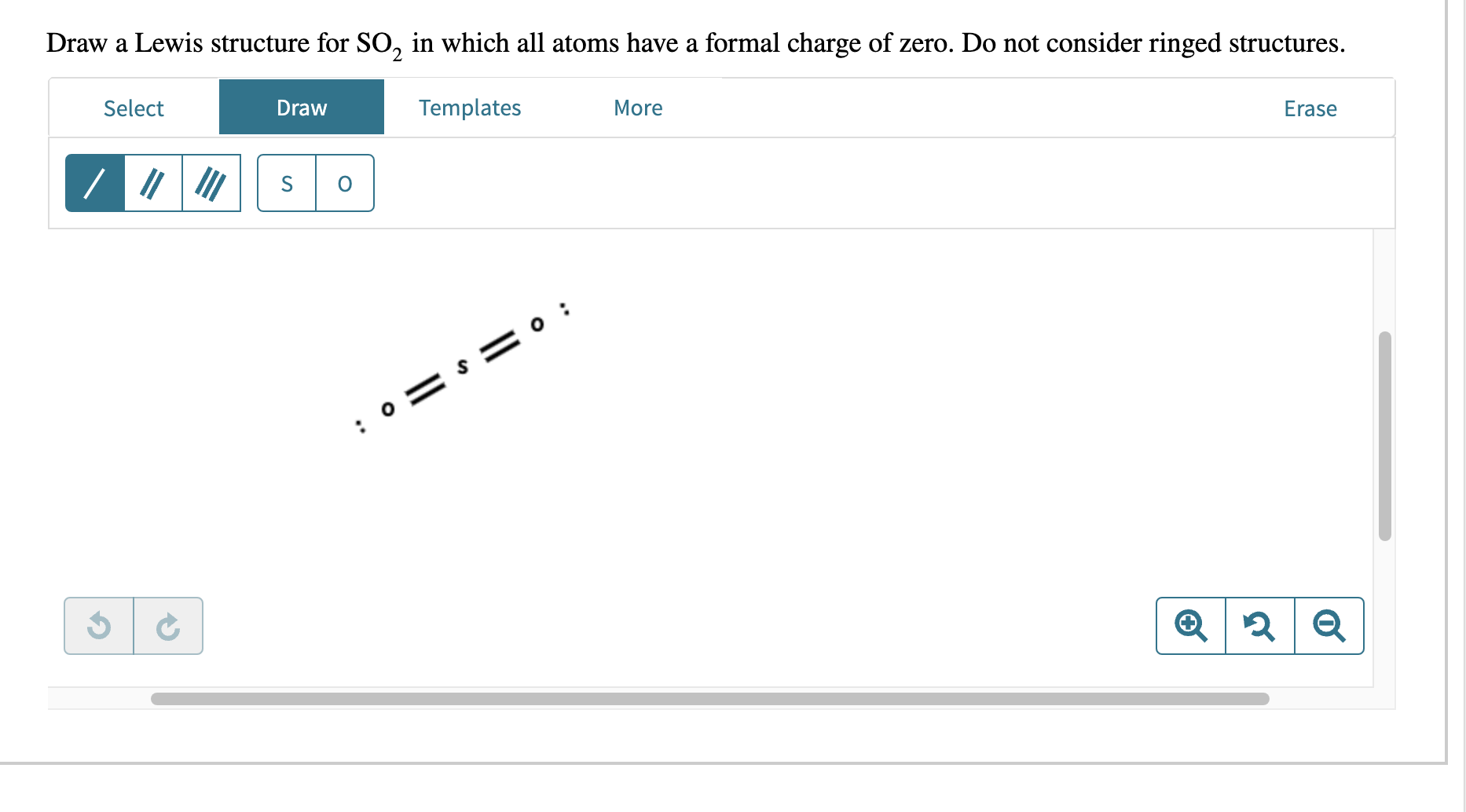
Draw the resonance structure of the enolate ion. Draw a Lewis structure for \( \mathrm{SO}_{2} \) in which all atoms obey the octet rule. Show formal charges. Do not consider ringed structures. Draw a Lewis structure for \( \mathrm{SO}_{2} \) in which all atoms have a formal charge of zero. Do not consider ringed structures.