Home /
Expert Answers /
Chemistry /
e-chatgpt-concludes-that-increasing-the-number-of-fluorine-atoms-attached-to-the-alpha-carb-pa207
(Solved): E. ChatGPT concludes that increasing the number of fluorine atoms attached to the \( \alpha \)-carb ...
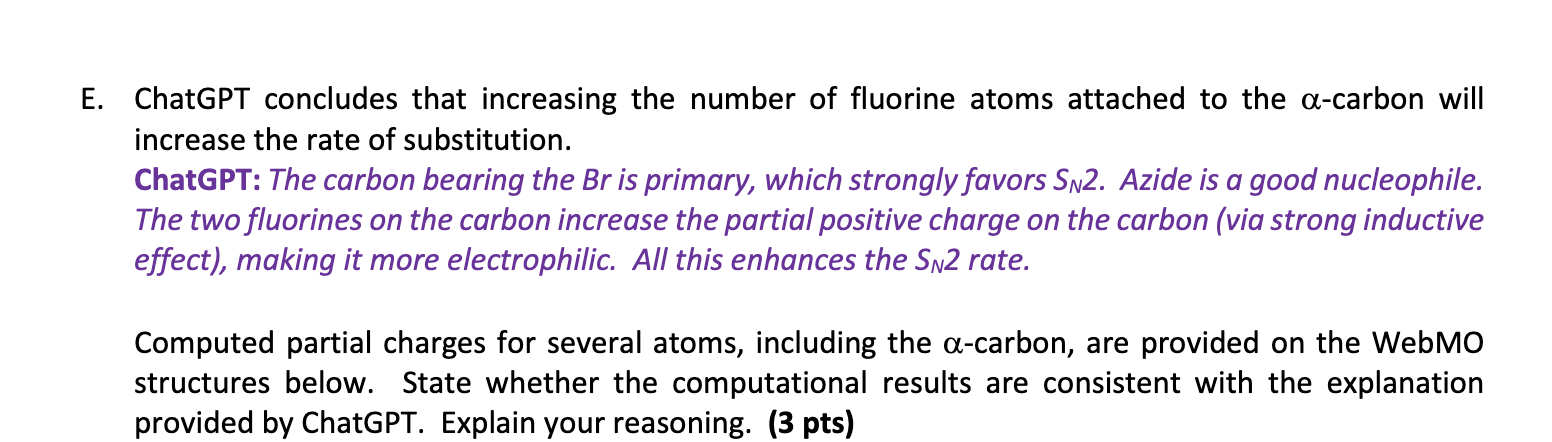
E. ChatGPT concludes that increasing the number of fluorine atoms attached to the \( \alpha \)-carbon will increase the rate of substitution. ChatGPT: The carbon bearing the Br is primary, which strongly favors \( S_{N} 2 \). Azide is a good nucleophile. The two fluorines on the carbon increase the partial positive charge on the carbon (via strong inductive effect), making it more electrophilic. All this enhances the \( S_{N} 2 \) rate. Computed partial charges for several atoms, including the \( \alpha \)-carbon, are provided on the WebMO structures below. State whether the computational results are consistent with the explanation provided by ChatGPT. Explain your reasoning. (3 pts)