Home /
Expert Answers /
Chemistry /
feedback-ent-attribution-jestion-6-1-point-hanoic-acid-ch-3-c-o-o-h-is-a-weak-acid-and-disso-pa120
(Solved): FEEDBACK ent attribution JESTION 6 - 1 POINT hanoic acid, CH_(3)(C)/(O)O H, is a weak acid and disso ...
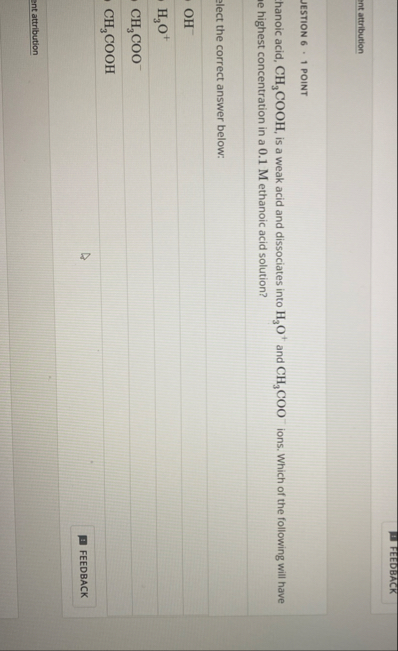
FEEDBACK ent attribution JESTION 6 - 1 POINT hanoic acid,
CH_(3)(C)/(O)O H, is a weak acid and dissociates into
H_(3)O^( )and (
CH_(3)(C)/(O)O ^(()
-)ions. Which of the following will have e highest concentration in a 0.1 M ethanoic acid solution? lect the correct answer below:
OH^(-)
H_(3)O^( )(
CH_(3)(C)/(O)O ^(()
-)
CH_(3)(C)/(O)O H