Home /
Expert Answers /
Chemistry /
for-each-chemical-reaction-in-the-table-below-decide-whether-the-highlighted-reactant-is-a-bronsted-pa525
(Solved): For each chemical reaction in the table below, decide whether the highlighted reactant is a Bronsted ...
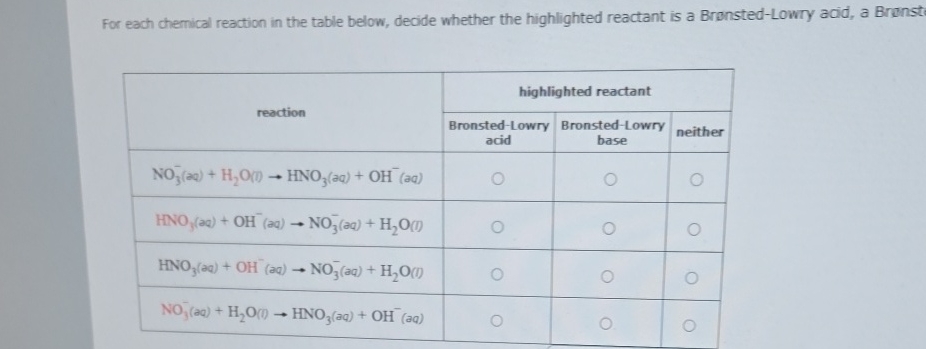
For each chemical reaction in the table below, decide whether the highlighted reactant is a Bronsted-Lowry acid, a Bronst: \table[[reaction,highlighted reactant],[Bronsted-Lowry acid,Bronsted-Lowry base,neither],[
NO_(3)^(-)(aq)+H_(2)O(0)->HNO_(3)(aq)+OH^(-)(aq),,,],[
HNO_(3)(aq)+OH^(-)(aq)->NO_(3)^(-)(aq)+H_(2)O(t),,,],[
HNO_(3)(aq)+OH^(-)(aq)->NO_(3)^(-)(aq)+H_(2)O(l),,,],[
NO_(3)^(-)(aq)+H_(2)O(0)->HNO_(3)(aq)+OH^(-)(aq),
◯,
◯,]]