Home /
Expert Answers /
Chemistry /
for-the-following-electrochemical-reaction-begin-array-ll-mathrm-al-3-mathrm-aq-3-m-pa951
(Solved): For the following electrochemical reaction: \[ \begin{array}{ll} \mathrm{Al}^{3+}(\mathrm{aq})+3 \m ...
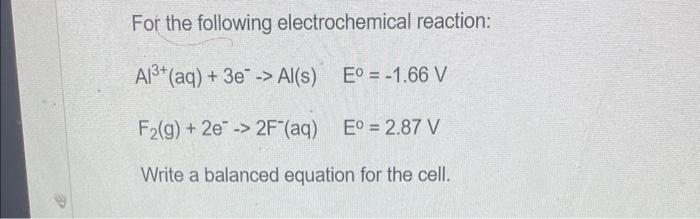
For the following electrochemical reaction: \[ \begin{array}{ll} \mathrm{Al}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Al}(\mathrm{s}) & E^{0}=-1.66 \mathrm{~V} \\ \mathrm{~F}_{2}(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-}(\mathrm{aq}) & E^{0}=2.87 \mathrm{~V} \end{array} \] Write a balanced equation for the cell.
Expert Answer
Ans- Given - Al3+(aq) + 3e- -> Al(s) Eo = -1.66 V F2(g)