Home /
Expert Answers /
Chemistry /
for-the-following-reaction-6-77-grams-of-oxygen-gas-are-mixed-with-excess-carbon-monoxide-t-pa247
(Solved): For the following reaction, \( 6.77 \) grams of oxygen gas are mixed with excess carbon monoxide. T ...
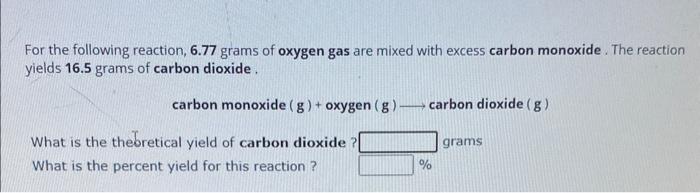
For the following reaction, \( 6.77 \) grams of oxygen gas are mixed with excess carbon monoxide. The reaction yields \( 16.5 \) grams of carbon dioxide . \[ \text { carbon monoxide }(\mathrm{g})+\operatorname{oxygen}(\mathrm{g}) \longrightarrow \text { carbon dioxide }(\mathrm{g}) \] What is the theoretical yield of carbon dioxide? grams What is the percent yield for this reaction ? \( \% \)