Home /
Expert Answers /
Chemistry /
formulas-q-mcsat-areaction-qsolution-0-ahrxn-greaction-molesreaction-consider-the-dissolu-pa515
(Solved): Formulas: q = MCSAT areaction + qsolution = 0 AHrxn = greaction/molesreaction Consider the dissolu ...
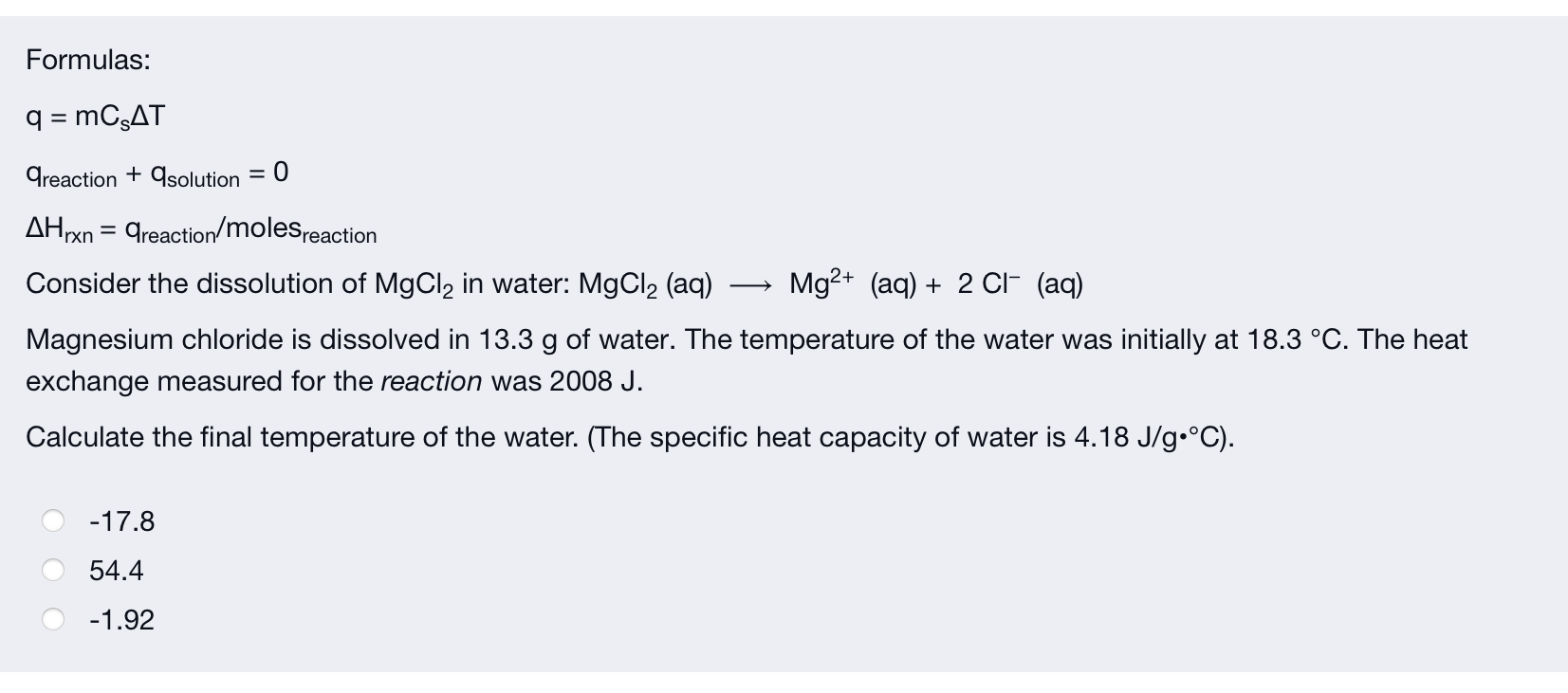
Formulas: q = MCSAT areaction + qsolution = 0 AHrxn = greaction/molesreaction Consider the dissolution of MgCl? in water: MgCl? (aq) Mg2+ (aq) + 2 CI- (aq) Magnesium chloride is dissolved in 13.3 g of water. The temperature of the water was initially at 18.3 °C. The heat exchange measured for the reaction was 2008 J. Calculate the final temperature of the water. (The specific heat capacity of water is 4.18 J/g °C). -17.8 54.4 -1.92