Home /
Expert Answers /
Chemistry /
given-that-the-reaction-mathrm-n-2-mathrm-g-2-mathrm-o-2-mathrm-g-rightarrow-2-pa550
(Solved): Given that the reaction \( \mathrm{N}_{2}(\mathrm{~g})+2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 ...
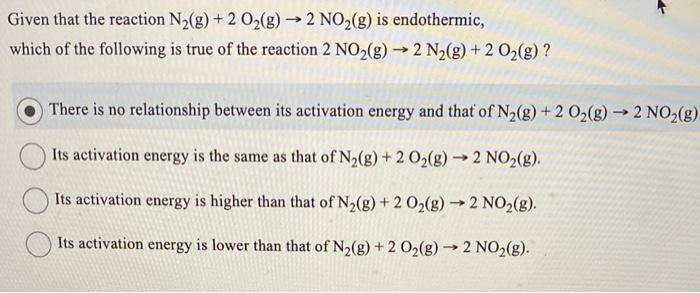
Given that the reaction \( \mathrm{N}_{2}(\mathrm{~g})+2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{NO}_{2}(\mathrm{~g}) \) is endothermic, which of the following is true of the reaction \( 2 \mathrm{NO}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{~N}_{2}(\mathrm{~g})+2 \mathrm{O}_{2}(\mathrm{~g}) \) ? There is no relationship between its activation energy and that of \( \mathrm{N}_{2}(\mathrm{~g})+2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{NO}_{2}(\mathrm{~g}) \) Its activation energy is the same as that of \( \mathrm{N}_{2}(\mathrm{~g})+2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{NO}_{2}(\mathrm{~g}) \). Its activation energy is higher than that of \( \mathrm{N}_{2}(\mathrm{~g})+2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{NO}_{2}(\mathrm{~g}) \). Its activation energy is lower than that of \( \mathrm{N}_{2}(\mathrm{~g})+2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{NO}_{2}(\mathrm{~g}) \).