Home /
Expert Answers /
Chemistry /
h-2-o-2-2hclolongrightarrowo-2-cl-2-2h-2-o-in-the-above-redox-reaction-use-oxidation-number-pa126
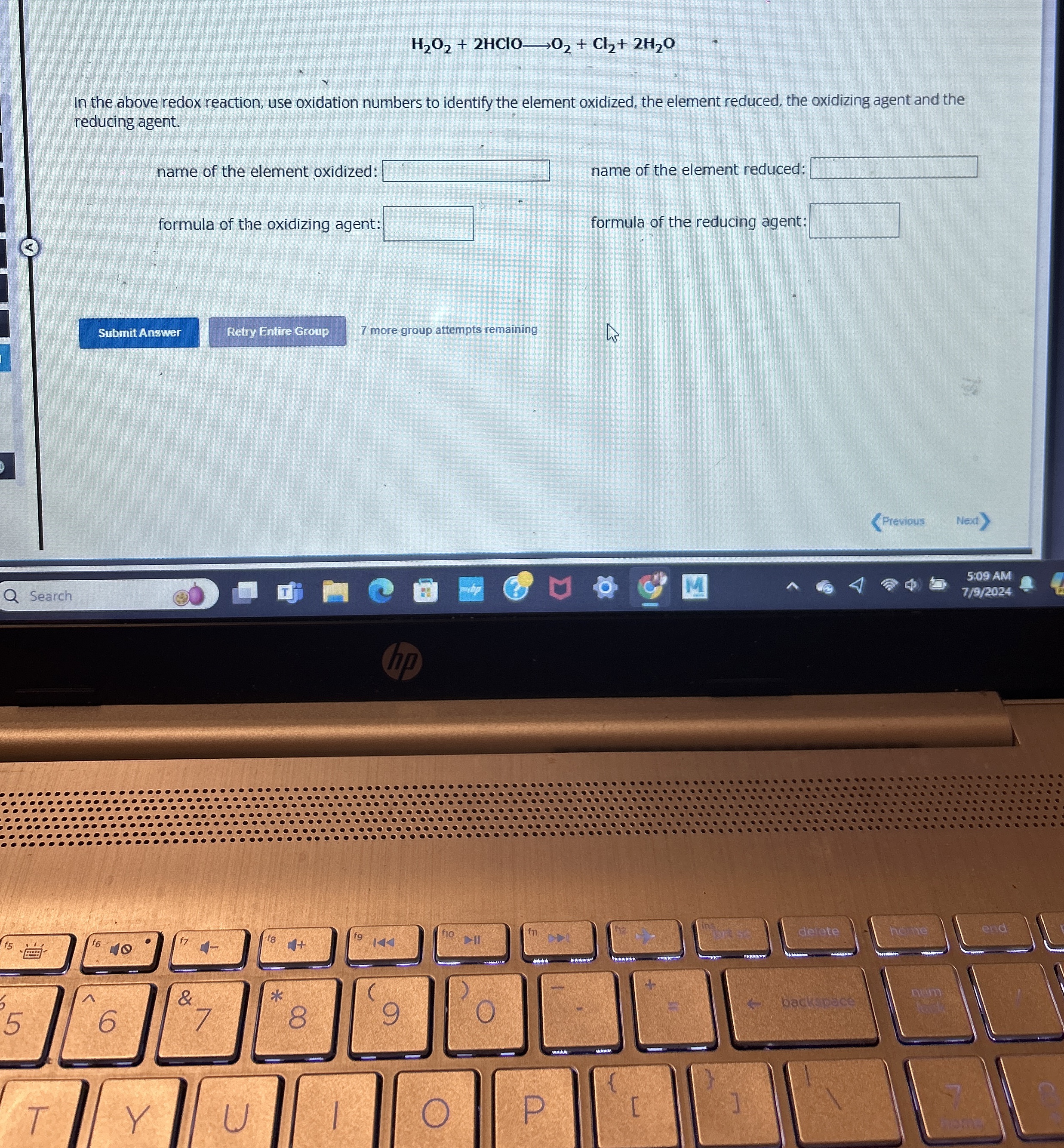
(Solved): H_(2)O_(2)+2HClOlongrightarrowO_(2)+Cl_(2)+2H_(2)O In the above redox reaction, use oxidation number ...
H_(2)O_(2)+2HClOlongrightarrowO_(2)+Cl_(2)+2H_(2)OIn the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent. name of the element oxidized:
◻name of the element reduced: formula of the oxidizing agent:
◻formula of the reducing agent
◻
◻7 more group attempts remaining Previous Next 509 AM
(7)/(9)/2024Search