Home /
Expert Answers /
Chemistry /
help-change-at-other-temperatures-gt-can-also-be-obtained-by-utilizing-this-equation-and-ass-pa850
(Solved): help change at other temperatures, GT, can also be obtained by utilizing this equation and ass ...
help
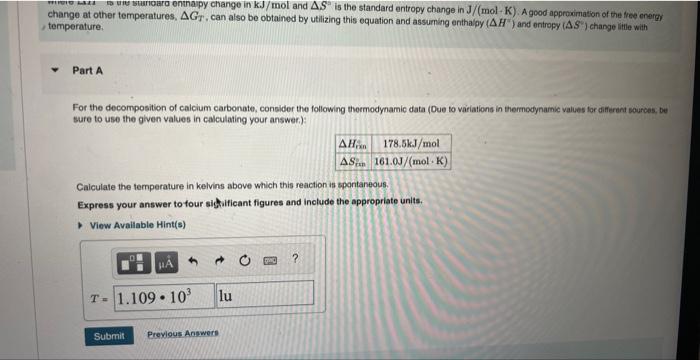


change at other temperatures, , can also be obtained by utilizing this equation and assuming enthalpy and entropy change little with temperature. Part A For the decomposition of calcium carbonate, consider the following thermodynamic data (Due to variations in themodynamic values for different sources, be sure to use the given values in calculating your answer.): Calculate the temperature in kelvins above which this reaction is spontaneous. Express your answer to tour sighificant figures and include the appropriate units.
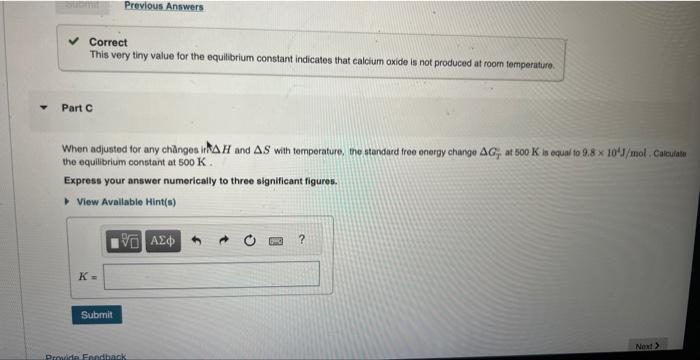
Correct This very tiny value for the equilibrium constant indicates that calclum axide is not produced at room temperature. Part C When adjusted for any chänges ift and with temperature. the standard free energy change at is equal to mol . Calculate the equilibrium constant at . Express your answer numerically to three significant figures.