Home /
Expert Answers /
Chemistry /
help-fast-please-question-6-determine-the-mass-in-grams-of-al2-so4-3-that-is-produced-when-486-ml-of-pa401
(Solved): help fast please QUESTION 6 Determine the mass in grams of Al2(SO4)3 that is produced when 486 mL of ...
help fast please
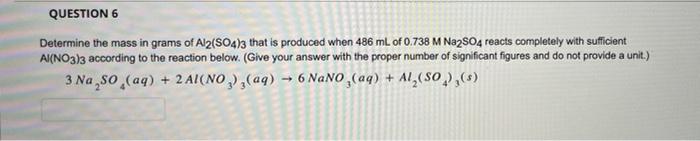
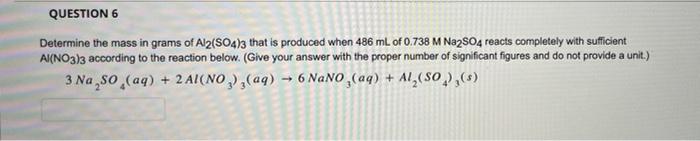
QUESTION 6 Determine the mass in grams of Al2(SO4)3 that is produced when 486 mL of 0.738 M Na2SO4 reacts completely with sufficient AI(NO3)3 according to the reaction below. (Give your answer with the proper number of significant figures and do not provide a unit.) 3 Na?SO (aq) + 2Al(NO3)3(aq) ?6 NaNO,(aq) + Al?(SO),(s)
Expert Answer
? Given for Na2SO4 Molarity = 0.738 M or mol/L Volume = 486 mL = 0.486 L Mole = Molarity × Volume Moles of Na2SO4