Home /
Expert Answers /
Chemistry /
how-3-given-that-the-ka-of-acetic-acid-is-1-698-x-10-5-what-is-the-concentration-of-h-ions-in-a-pa714
(Solved): how? 3. Given that the Ka of acetic acid is 1.698 x 10-5, what is the concentration of H* ions in a ...
how?
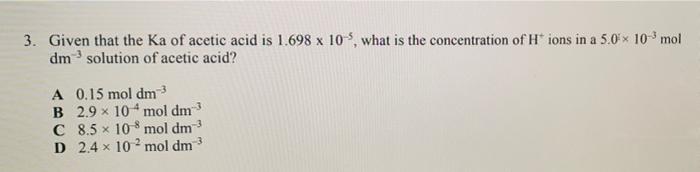
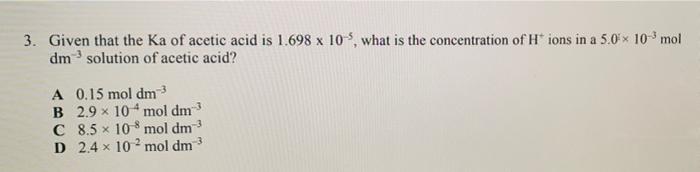
3. Given that the Ka of acetic acid is 1.698 x 10-5, what is the concentration of H* ions in a 5.0× 10-³ mol dm solution of acetic acid? A 0.15 mol dm-³ B 2.9 x 10 mol dm³ 8.5 x 108 mol dm-³ C D 2.4 x 102 mol dm³