Home /
Expert Answers /
Chemistry /
how-many-moles-of-gas-are-there-in-the-container-remember-to-use-three-significant-figures-for-al-pa779
(Solved): How many moles of gas are there in the container? Remember to use three significant figures for al ...
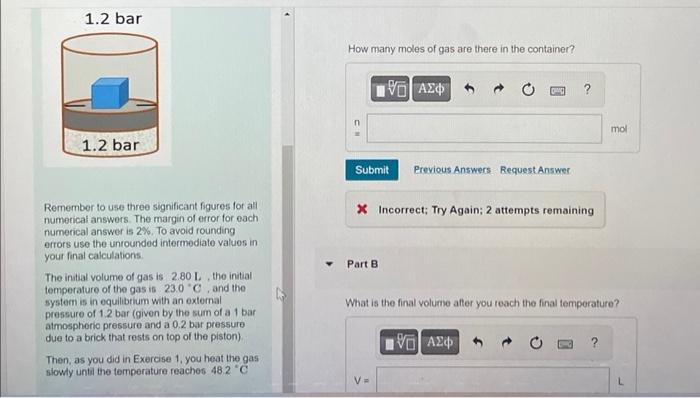
How many moles of gas are there in the container? Remember to use three significant figures for al numerical answers. The margin of error for each numerical answer is \( 2 \% \). To avoid rounding errors use the unrounded intermediate values in your final calculations. * Incorrect; Try Again; 2 attempts remaining The inutial volume of gas is \( 2.80 \mathrm{~L} \), the initial temperature of the gas is \( 23.0^{\circ} \mathrm{C} \), and the system is in equilibrium with an extemal Part B pressure of \( 1.2 \) bar (given by the sum of a 1 bar atmospherio pressure and a \( 0.2 \) bar pressure due to a brick that rests on top of the piston) What is the final volume after you reach the final temperature? Then, as you did in Exercise 1, you heat the gas siowly until the temperature reachos \( 48.2^{\circ} \mathrm{C} \)
What is the final pressure after you reach the final temperature? Part D What is the sign of \( w \) ? zero positive negative
What is the value of \( w \) ?