Home /
Expert Answers /
Chemistry /
hybrid-orbitals-hybrid-orbitals-are-formed-by-combining-the-valence-orbitals-on-an-atom-here-are-pa560
(Solved): Hybrid Orbitals Hybrid orbitals are formed by combining the valence orbitals on an atom. here are ...
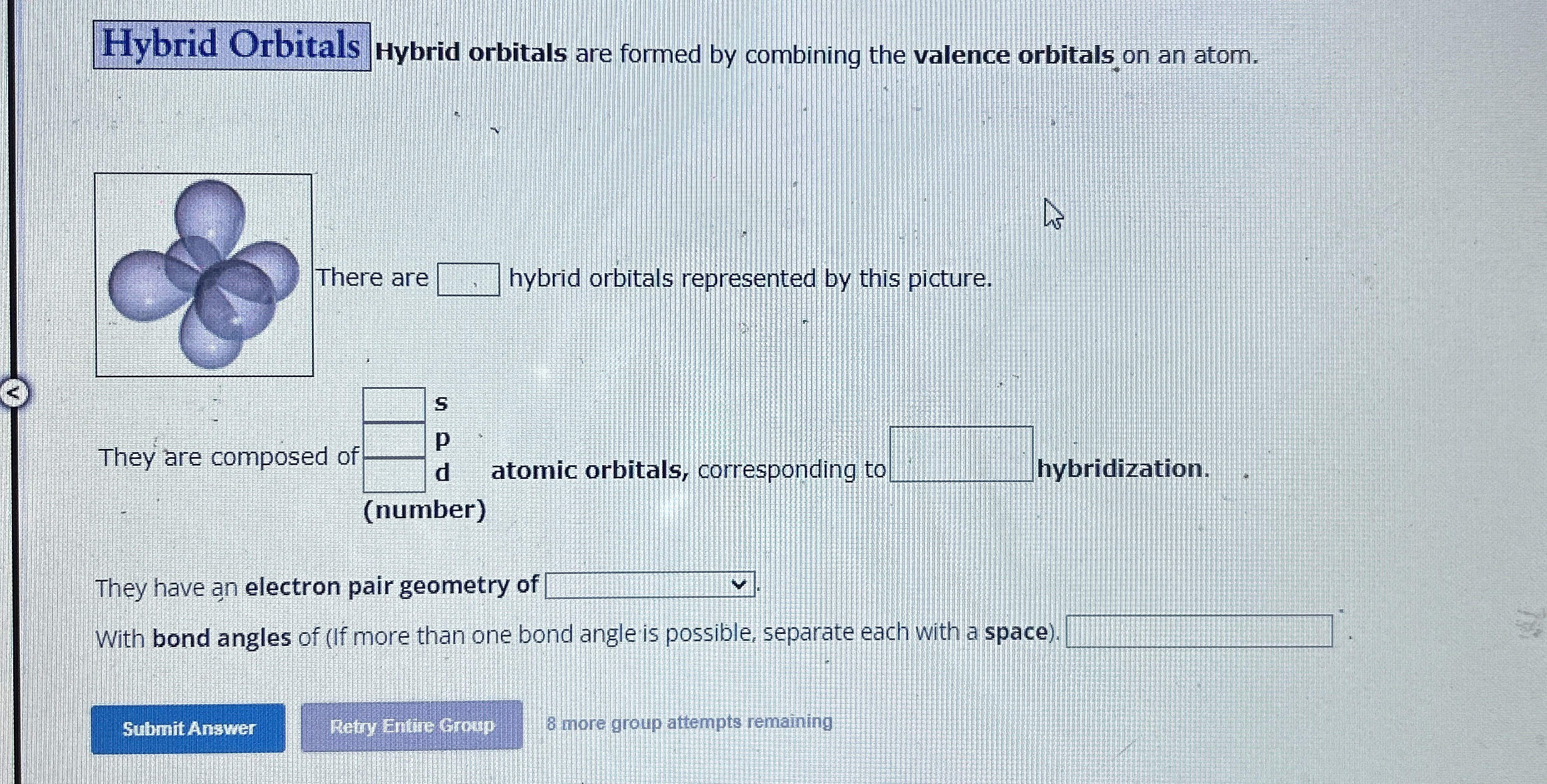
Hybrid Orbitals Hybrid orbitals are formed by combining the valence orbitals on an atom. here are
◻hybrid orbitals represented by this picture. They are composed of
|[ s ],[ (number) atomic orbitals, corresponding to ]||
◻hybridization. They have an electron pair geometry of
◻With bond angles of (If more than one bond angle is possible, separate each with a space)
◻
◻
◻8 more group attempts remaining