Home /
Expert Answers /
Chemistry /
if-25ml-of-a-3m-naoh-solution-ph-14-is-mixed-with-25ml-of-dcm-containing-aspirin-and-the-resul-pa924
(Solved): If 25mL of a 3M NaOH solution (pH = 14) is mixed with 25mL of DCM containing aspirin, and the resul ...
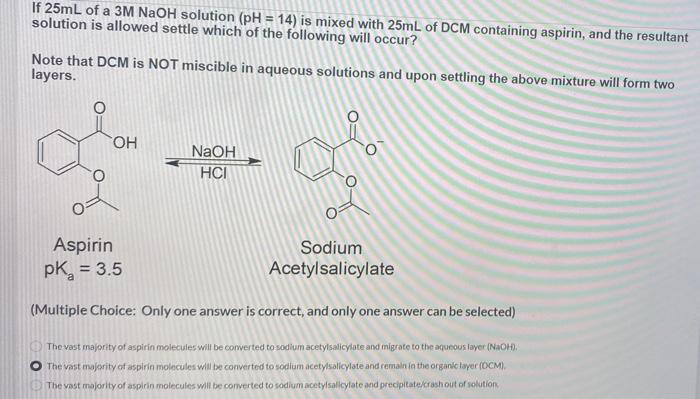
If 25mL of a 3M NaOH solution (pH = 14) is mixed with 25mL of DCM containing aspirin, and the resultant solution is allowed settle which of the following will occur? Note that DCM is NOT miscible in aqueous solutions and upon settling the above mixture will form two layers. OH NaOH HCI Aspirin pk = 3.5 Sodium Acetylsalicylate (Multiple Choice: Only one answer is correct, and only one answer can be selected) The vast majority of aspirin molecules will be converted to sodium acetylsalicylate and migrate to the aqueous layer (NaOH). The vast majority of aspirin molecules will be converted to sodium acetylsalicylate and remain in the organic layer (DCM). The vast majority of aspirin molecules will be converted to sodium acetylsalicylate and precipitate/crash out of solution. O O
Expert Answer
The correct Answer is The vast majority of aspirin molecule will b