Home /
Expert Answers /
Biology /
if-a-more-nbsp-a-if-a-more-concentrated-initial-solution-of-sodium-bicarbonate-was-used-in-be-c-w-pa803
(Solved): if a more a. If a more concentrated initial solution of sodium bicarbonate was used in be C, w ...
if a more



a. If a more concentrated initial solution of sodium bicarbonate was used in be C, would it require more or less bicarbonate to neutralize the acid? Why?
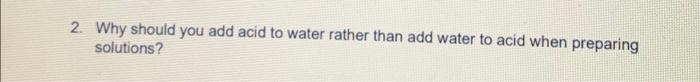
2. Why should you add acid to water rather than add water to acid when preparing solutions?
Expert Answer
a. If the initial soluntion is more concentrated that means there are high number of molecules of bicarbonate ions in the solution making the ent