Home /
Expert Answers /
Chemistry /
if-a-red-blood-cell-was-placed-in-a-hypertonic-solution-which-of-the-following-statements-is-tr-pa821
(Solved): If a red blood cell was placed in a hypertonic solution, which of the following statements is tr ...
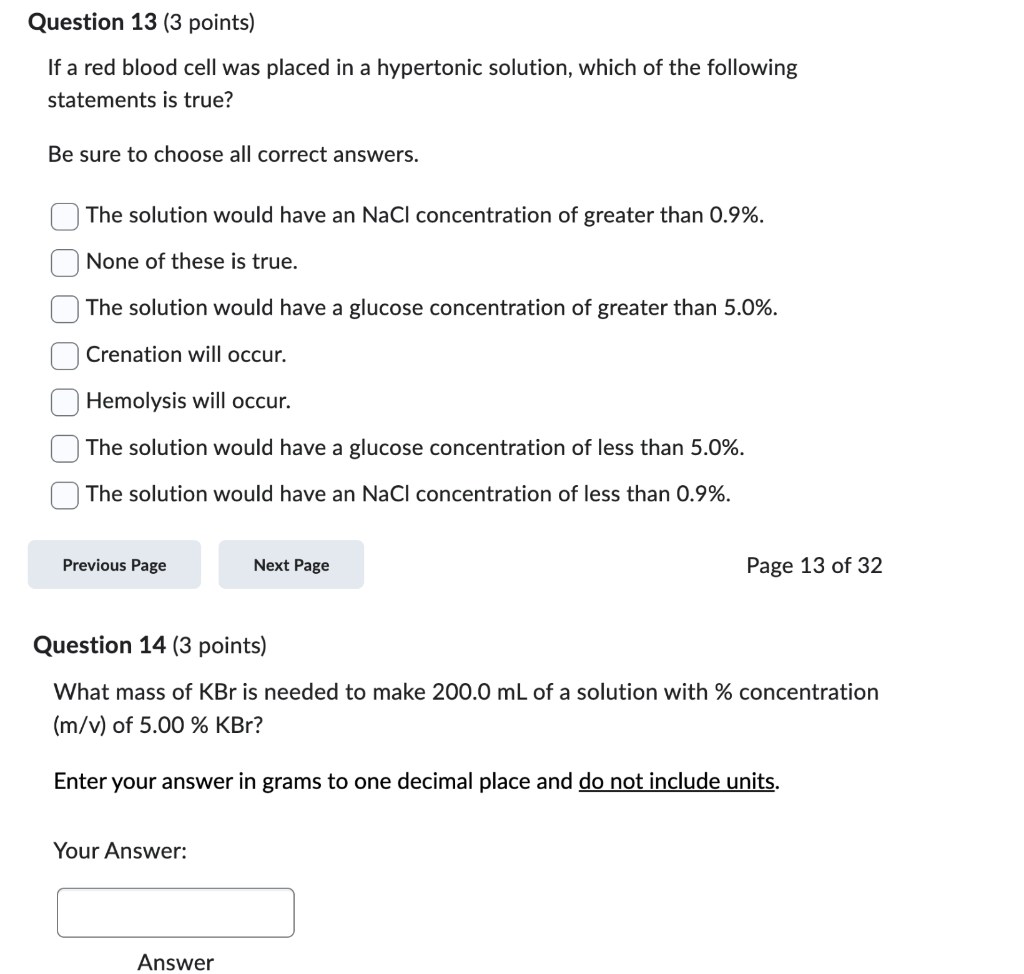
If a red blood cell was placed in a hypertonic solution, which of the following statements is true? Be sure to choose all correct answers. The solution would have an \( \mathrm{NaCl} \) concentration of greater than \( 0.9 \% \). None of these is true. The solution would have a glucose concentration of greater than 5.0\%. Crenation will occur. Hemolysis will occur. The solution would have a glucose concentration of less than \( 5.0 \% \). The solution would have an \( \mathrm{NaCl} \) concentration of less than \( 0.9 \% \). Page 13 of 32 Question 14 (3 points) What mass of \( \mathrm{KBr} \) is needed to make \( 200.0 \mathrm{~mL} \) of a solution with \% concentration \( (\mathrm{m} / \mathrm{v}) \) of \( 5.00 \% \mathrm{KBr} \) ? Enter your answer in grams to one decimal place and do not include units. Your Answer: Answer
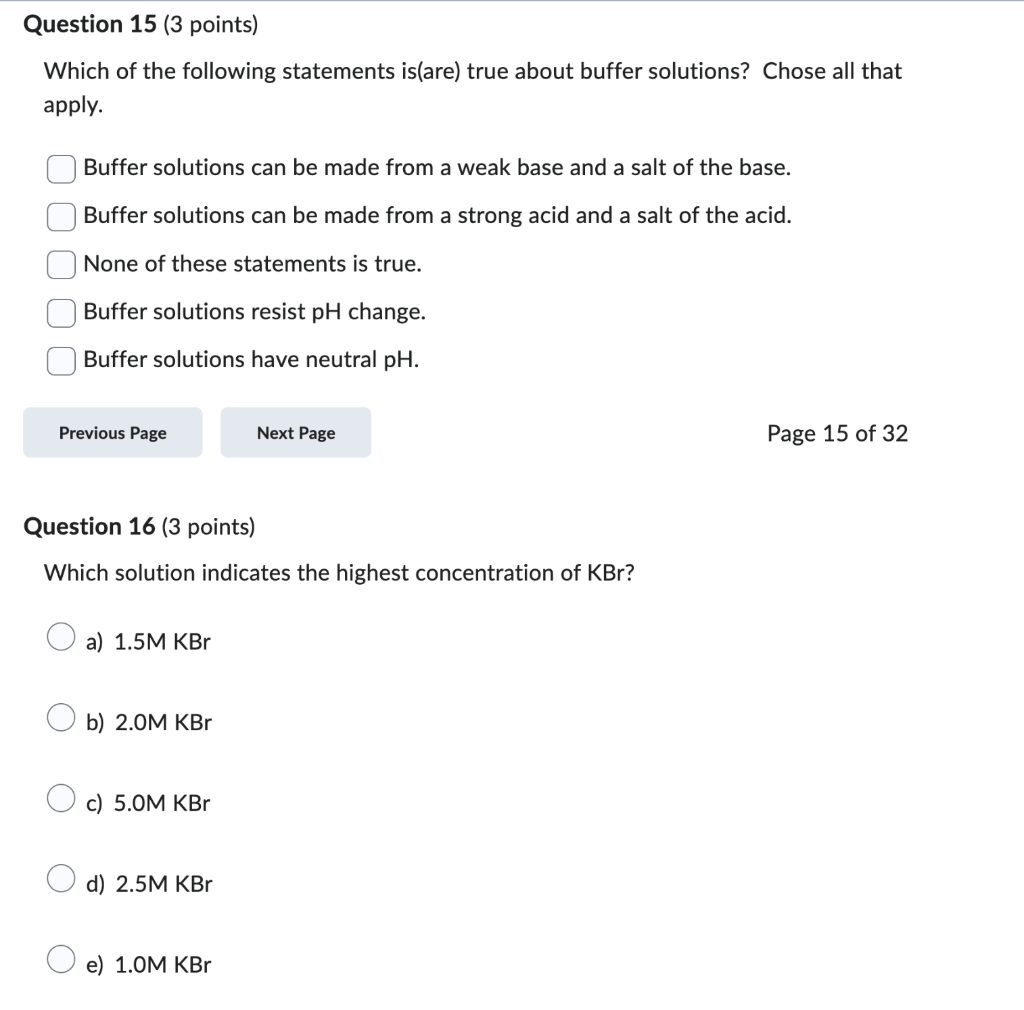
Which of the following statements is(are) true about buffer solutions? Chose all that apply. Buffer solutions can be made from a weak base and a salt of the base. Buffer solutions can be made from a strong acid and a salt of the acid. None of these statements is true. Buffer solutions resist \( \mathrm{pH} \) change. Buffer solutions have neutral \( \mathrm{pH} \). Page 15 of 32 Question 16 ( 3 points) Which solution indicates the highest concentration of \( \mathrm{KBr} \) ? a) \( 1.5 \mathrm{M} \mathrm{KBr} \) b) \( 2.0 \mathrm{M} \mathrm{KBr} \) c) \( 5.0 \mathrm{M} \mathrm{KBr} \) d) \( 2.5 \mathrm{M} \mathrm{KBr} \) e) \( 1.0 \mathrm{M} \mathrm{KBr} \)
Expert Answer
Ans 13. Red blood cells have 0.9% NaCl so the hypertonic solution should have NaCl greater than 0.9% and glucose concentration le