Home /
Expert Answers /
Biology /
if-supplied-with-surfactant-solutions-at-a-concentration-of-50-mm-calculate-how-much-of-the-stock-s-pa585
(Solved): If supplied with surfactant solutions at a concentration of 50 mM, calculate how much of the stock s ...
If supplied with surfactant solutions at a concentration of 50 mM, calculate how much of the stock solution, and how much water you would need to make 5 mL of solution at the concentrations of 50 mM, 25 mM, 10 mM and 5 mM.
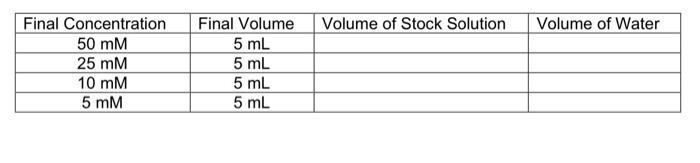
\begin{tabular}{|c|c|c|l|} \hline Final Concentration & Final Volume & Volume of Stock Solution & Volume of Water \\ \hline \( 50 \mathrm{mM} \) & \( 5 \mathrm{~mL} \) & & \\ \hline \( 25 \mathrm{mM} \) & \( 5 \mathrm{~mL} \) & & \\ \hline \( 10 \mathrm{mM} \) & \( 5 \mathrm{~mL} \) & & \\ \hline \( 5 \mathrm{mM} \) & \( 5 \mathrm{~mL} \) & & \\ \hline \end{tabular}
Expert Answer
We know that, V1C1 = V2C2 where, V1 = volume of stock solution, C1 = concentration of stock solution, V2 = volume of final solution, C2 = concentration of final solution According to the question, concentration of surfactant solution (C1) = 50