Home /
Expert Answers /
Chemistry /
iii-phosphorus-pentachloride-forms-molecular-dimers-in-ccl-4-solution-describe-and-draw-the-str-pa583
(Solved): (iii) Phosphorus pentachloride forms molecular dimers in CCl_(4) solution. Describe and draw the str ...
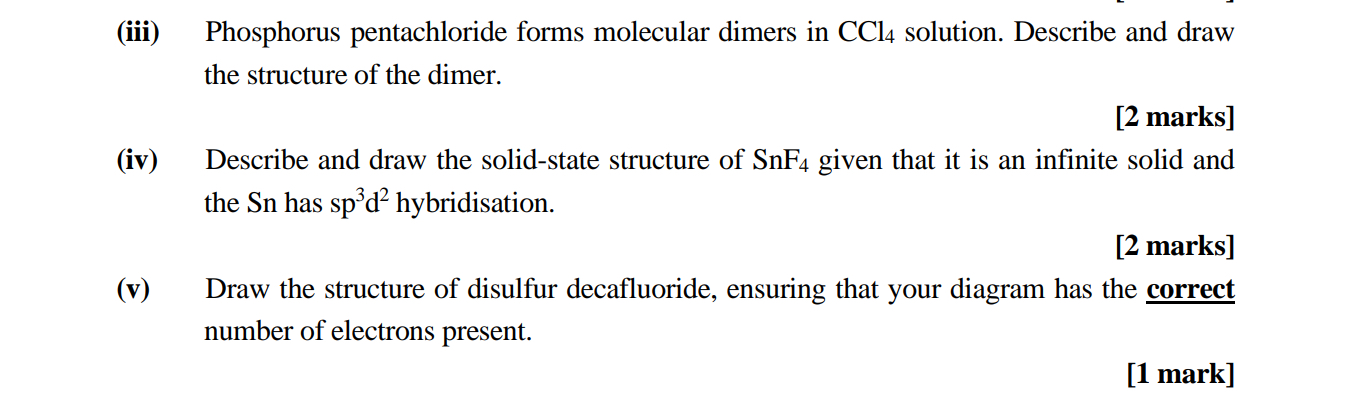
(iii) Phosphorus pentachloride forms molecular dimers in
CCl_(4)solution. Describe and draw the structure of the dimer. [2 marks] (iv) Describe and draw the solid-state structure of
SnF_(4)given that it is an infinite solid and the Sn has
sp^(3)d^(2)hybridisation. [2 marks] (v) Draw the structure of disulfur decafluoride, ensuring that your diagram has the correct number of electrons present. [1 mark]