Home /
Expert Answers /
Chemistry /
in-each-case-she-fills-a-reaction-vessel-with-some-mixture-of-the-reactants-and-products-at-a-const-pa430
(Solved): In each case, she fills a reaction vessel with some mixture of the reactants and products at a const ...
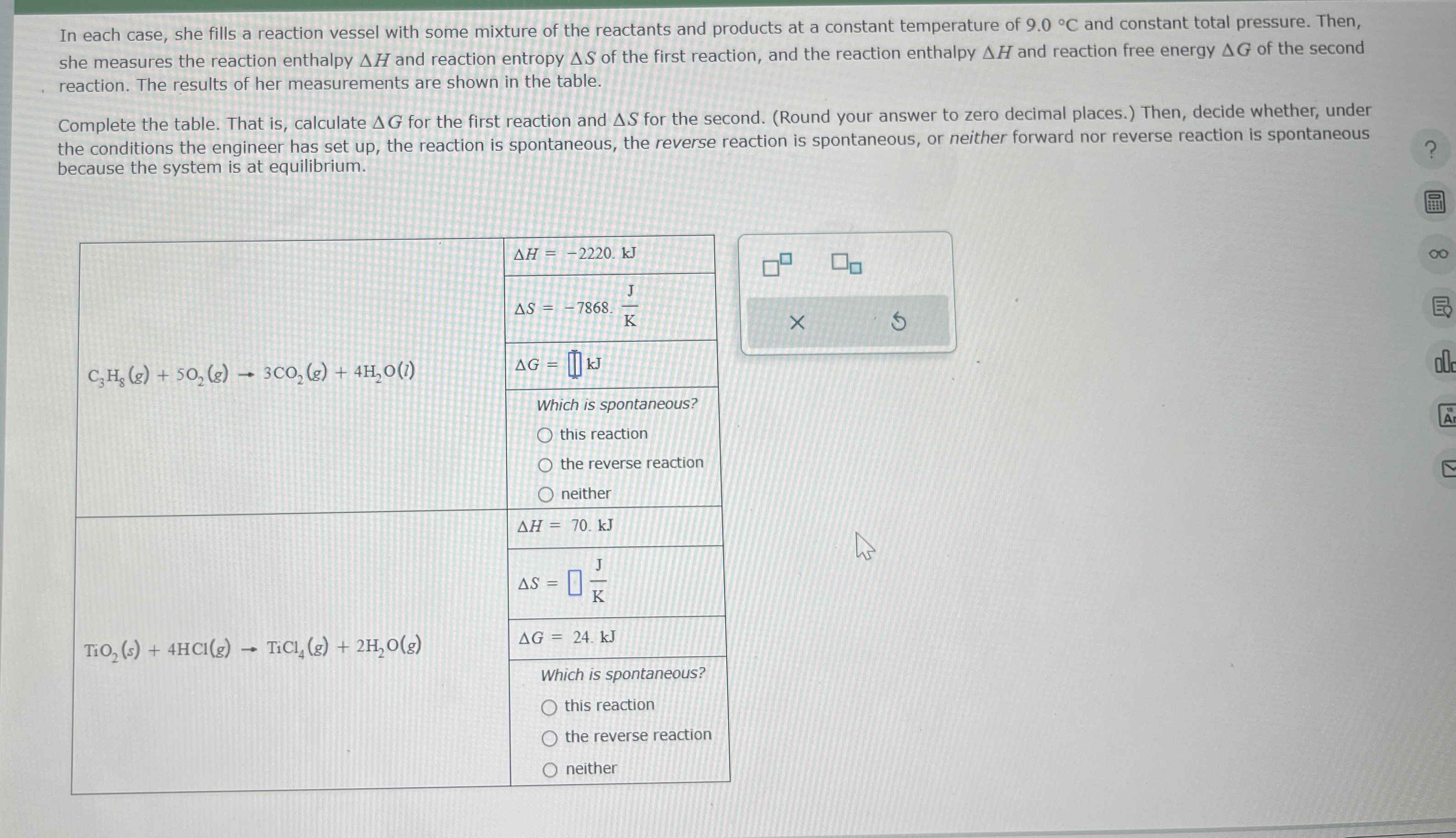
In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of
9.0\deg Cand constant total pressure. Then, she measures the reaction enthalpy
\Delta Hand reaction entropy
\Delta Sof the first reaction, and the reaction enthalpy
\Delta Hand reaction free energy
\Delta Gof the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate
\Delta Gfor the first reaction and
\Delta Sfor the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. \table[[
C_(3)H_(8)(g)+5O_(2)(g)->3CO_(2)(g)+4H_(2)O(l),
\Delta H=-2220.kJ