Home /
Expert Answers /
Statistics and Probability /
in-randomized-double-blind-clinical-trials-of-a-new-vaccine-rats-were-randomly-divided-into-two-g-pa495
(Solved): In randomized, double-blind clinical trials of a new vaccine, rats were randomly divided into two g ...
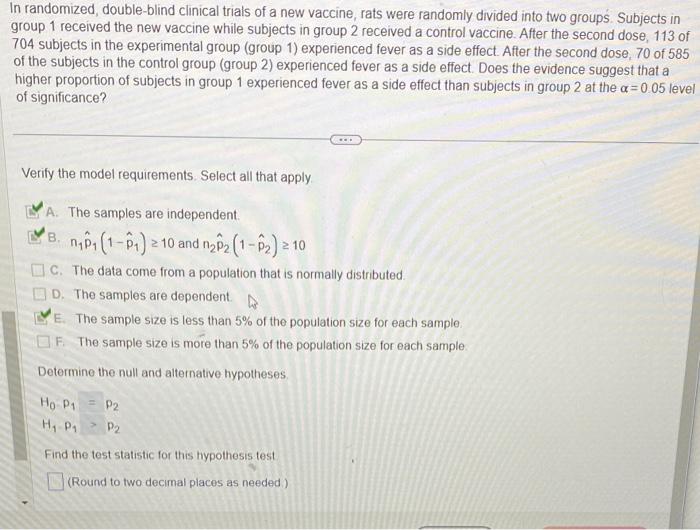
In randomized, double-blind clinical trials of a new vaccine, rats were randomly divided into two groups. Subjects in group 1 received the new vaccine while subjects in group 2 received a control vaccine. After the second dose, 113 of 704 subjects in the experimental group (group 1) experienced fever as a side effect. After the second dose, 70 of 585 of the subjects in the control group (group 2) experienced fever as a side effect. Does the evidence suggest that a higher proportion of subjects in group 1 experienced fever as a side effect than subjects in group 2 at the \( \alpha=0.05 \) level of significance? Verify the model requirements. Select all that apply. A. The samples are independent. B. \( n_{1} \hat{p}_{1}\left(1-\hat{p}_{1}\right) \geq 10 \) and \( n_{2} \hat{p}_{2}\left(1-\hat{p}_{2}\right) \geq 10 \) C. The data come from a population that is normally distributed. D. The samples are dependent. E. The sample size is less than \( 5 \% \) of the population size for each sample. F. The sample size is more than \( 5 \% \) of the population size for each sample. Determine the null and alternative hypotheses. \[ \begin{array}{l} H_{0} \cdot P_{1}=P_{2} \\ H_{1} \cdot P_{1}>P_{2} \end{array} \] Find the test statistic for this hypothesis test (Round to two decimal places as needed)
Expert Answer
Byusing STATCRUNCH > Go to Stat