(Solved): In this activity, you will make measurements using a virtual TLC plate. In thin-layer chromatography ...
In this activity, you will make measurements using a virtual TLC plate. In thin-layer chromatography, or TLC, compounds are separated based on their differential interactions between a stationary phase and a mobile phase. In normal phase TLC, the stationary phase is a thin layer of a polar material, such as silica, cellulose, or alumina, and the mobile phase is a less polar developing solvent which moves along the stationary phase of the TLC plate through capillary action. Since the stationary phase in normal phase TLC is polar, polar compounds have stronger interactions with the stationary phase than the developing solvent, so they will not travel as far along the stationary phase. Whereas less polar compounds will interact more strongly with the developing solvent and will travel further along the stationary phase. The distance traveled by a compound relative to the distance the developing solvent traveled is called the retention factor, or
R_(f), and is consistent for a compound provided the TLC conditions are consistent. The
R_(f)of an unknown compound can be compared to the
R_(f)of a known compound, to determine if they are likely to be the same compound or different compounds. Use the image provided below to practice determining
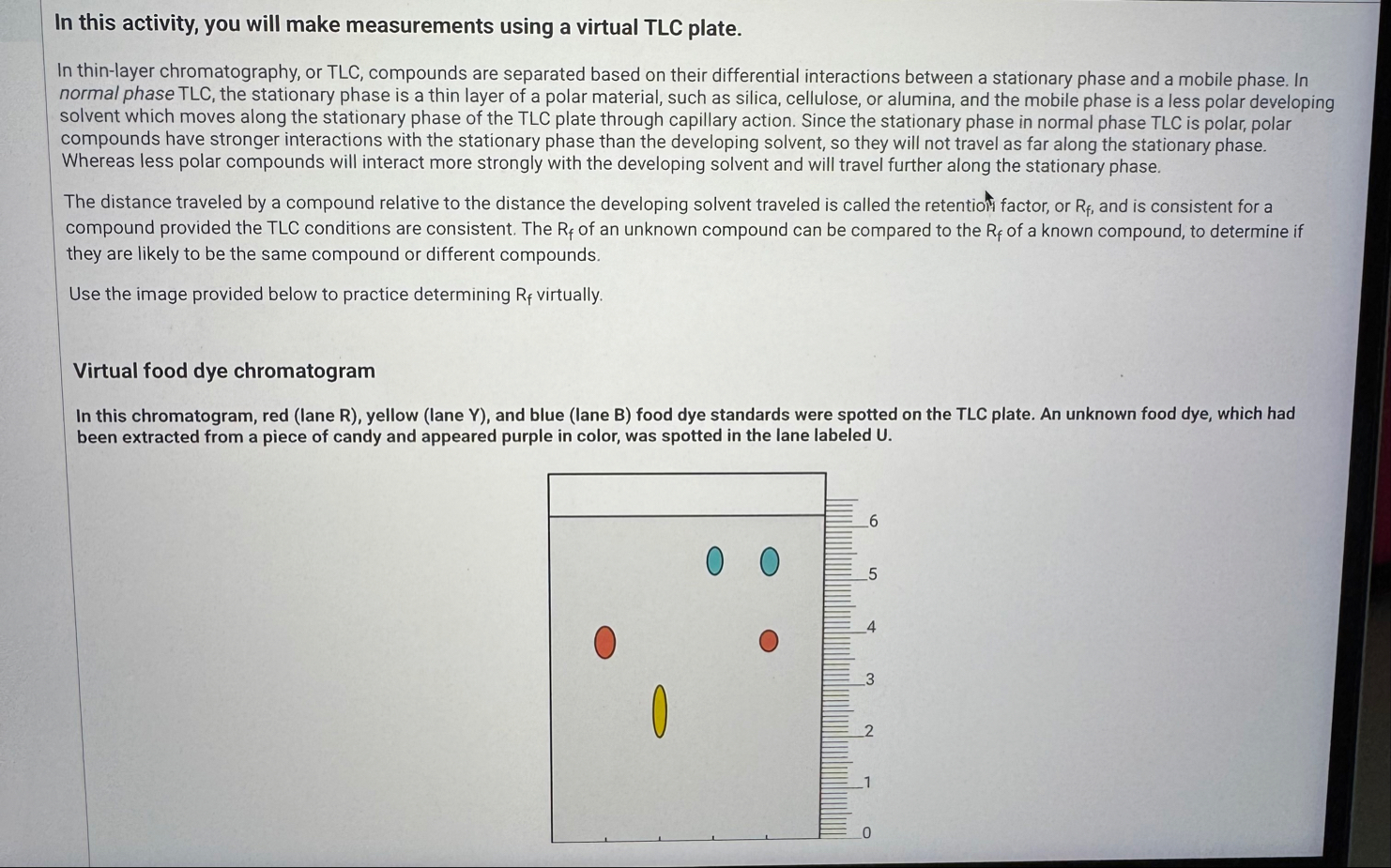
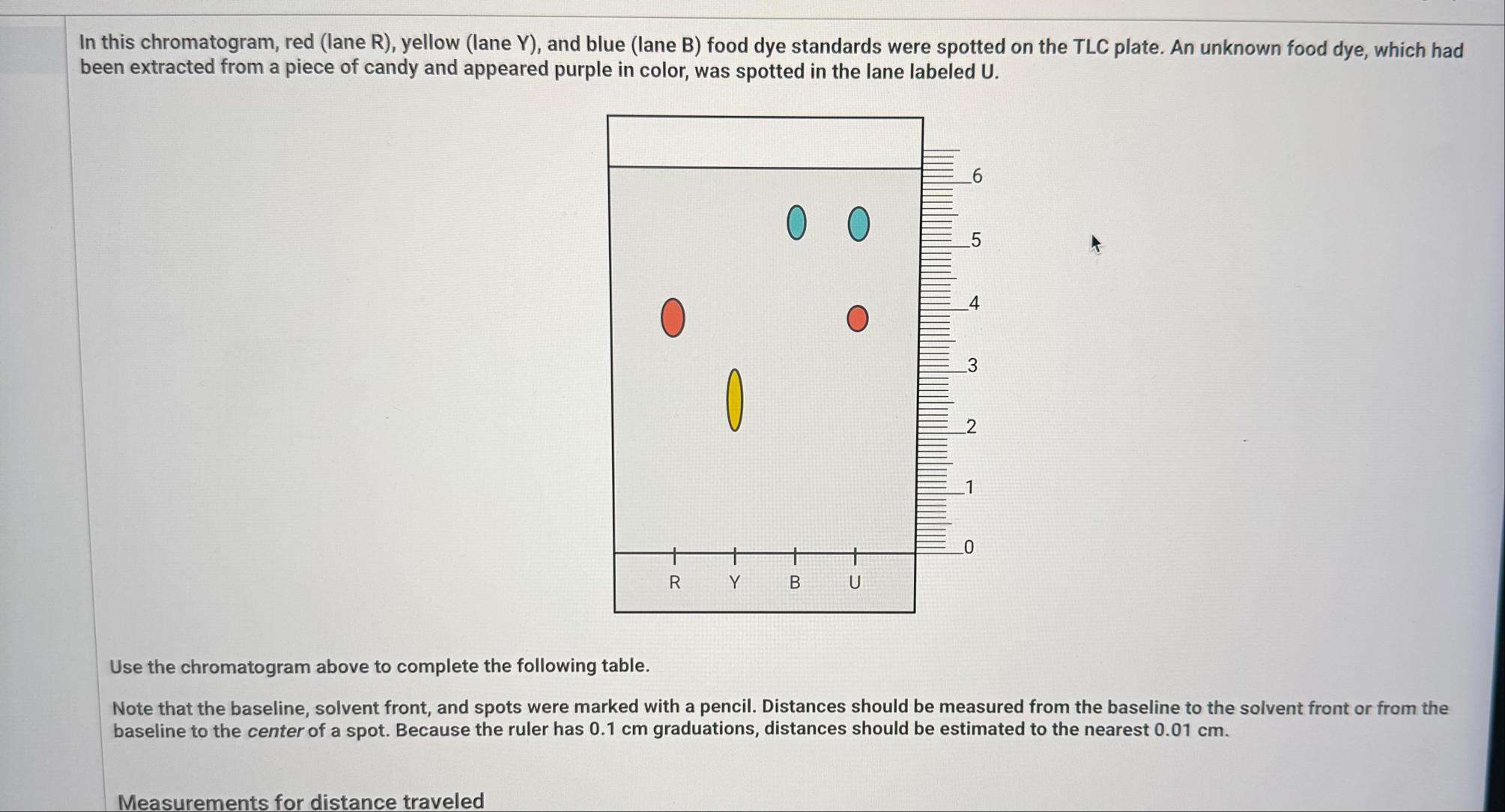
R_(f)virtually. Virtual food dye chromatogram In this chromatogram, red (lane R), yellow (lane Y), and blue (lane B) food dye standards were spotted on the TLC plate. An unknown food dye, which had been extracted from a piece of candy and appeared purple in color, was spotted in the lane labeled
U.In this chromatogram, red (lane R), yellow (lane Y), and blue (lane B) food dye standards were spotted on the TLC plate. An unknown food dye, which had been extracted from a piece of candy and appeared purple in color, was spotted in the lane labeled
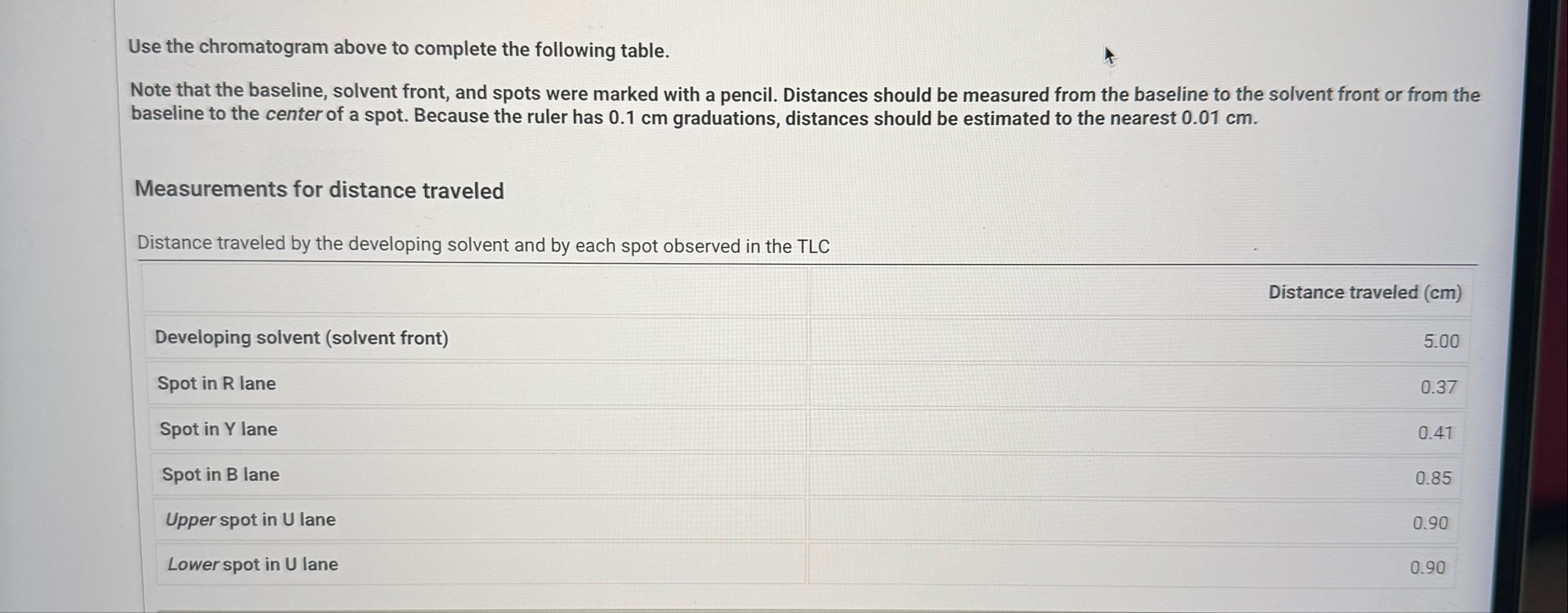
U. Use the chromatogram above to complete the following table. Note that the baseline, solvent front, and spots were marked with a pencil. Distances should be measured from the baseline to the solvent front or from the baseline to the center of a spot. Because the ruler has 0.1 cm graduations, distances should be estimated to the nearest 0.01 cm . Measurements for distance traveledUse the chromatogram above to complete the following table. Note that the baseline, solvent front, and spots were marked with a pencil. Distances should be measured from the baseline to the solvent front or from the baseline to the center of a spot. Because the ruler has 0.1 cm graduations, distances should be estimated to the nearest 0.01 cm . Measurements for distance traveled Distance traveled by the developing solvent and by each spot observed in the TLC \table[[,Distance traveled (cm)],[Developing solvent (solvent front),5.00],[Spot in R lane,0.37],[Spot in Y lane,0.41],[Spot in B lane,0.85],[Upper spot in U lane,0.90],[Lower spot in U lane,0.90]]