Home /
Expert Answers /
Chemistry /
in-which-thermochemical-equations-would-the-delta-h-be-considered-a-heat-of-solution-c-6-h-6-s-pa798
(Solved): In which thermochemical equations would the \Delta H be considered a heat of solution? C_(6)H_(6)(s) ...
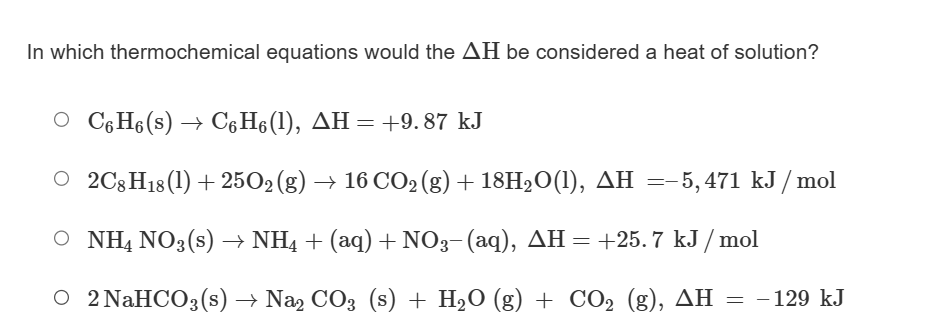
In which thermochemical equations would the \Delta H be considered a heat of solution?
C_(6)H_(6)(s)->C_(6)H_(6)(l),\Delta H=+9.87kJ
2C_(8)H_(18)(l)+25O_(2)(g)->16CO_(2)(g)+18H_(2)O(l),\Delta H=-5,471k(J)/(m)ol
NH_(4)NO_(3)(s)->NH_(4)+(aq)+NO_(3)-(aq),\Delta H=+25.7k(J)/(m)ol
2NaHCO_(3)(s)->Na_(2)CO_(3)(s)+H_(2)O(g)+CO_(2)(g),\Delta H=-129kJ