Home /
Expert Answers /
Chemistry /
indicate-the-concentration-of-each-ion-present-in-the-solution-formed-by-mixing-10-0-mathrm-ml-pa690
(Solved): Indicate the concentration of each ion present in the solution formed by mixing \( 10.0 \mathrm{~mL ...
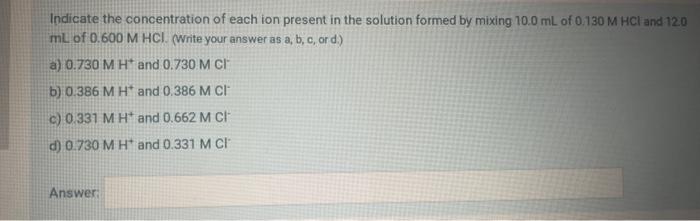
Indicate the concentration of each ion present in the solution formed by mixing \( 10.0 \mathrm{~mL} \) of \( 0.130 \mathrm{M} \mathrm{HCl} \) and 120 mL of \( 0.600 \mathrm{M} \mathrm{HCl} \). (Write your answer as \( a, b, c \), or d.) a) \( 0.730 \mathrm{M} \mathrm{H}^{+} \)and \( 0.730 \mathrm{M} \mathrm{Cl}^{-} \) b) \( 0.386 \mathrm{M} \mathrm{H}^{+} \)and \( 0.386 \mathrm{M} \mathrm{Cl}^{-} \) c) \( 0.331 \mathrm{MH}^{+} \)and \( 0.662 \mathrm{M} \mathrm{Cl}^{-} \) d) \( 0.730 \mathrm{M} \mathrm{H}^{+} \)and \( 0.331 \mathrm{M} \mathrm{Cl}^{-} \) Answ
Expert Answer
Given: The volume of 0.130 M HCl = 10.0 mL = 10.0 x 10-3 L = 0.01 L The molarity of first HCl solution = 0.130 M The volume of 0.600 M HCl = 12.0 mL = 12.0 x 10-3 L = 0.012 L The molarity of second HCl solution = 0.600 M Find: Find the concentration