Home /
Expert Answers /
Chemistry /
it-takes-17-5ml-of-a-0-250m-solution-of-naoh-to-neutralize-20-0ml-of-a-hcl-solution-of-an-unkno-pa397
(Solved): It takes 17.5mL of a 0.250M solution of NaOH to neutralize 20.0mL of a HCl solution of an unkno ...
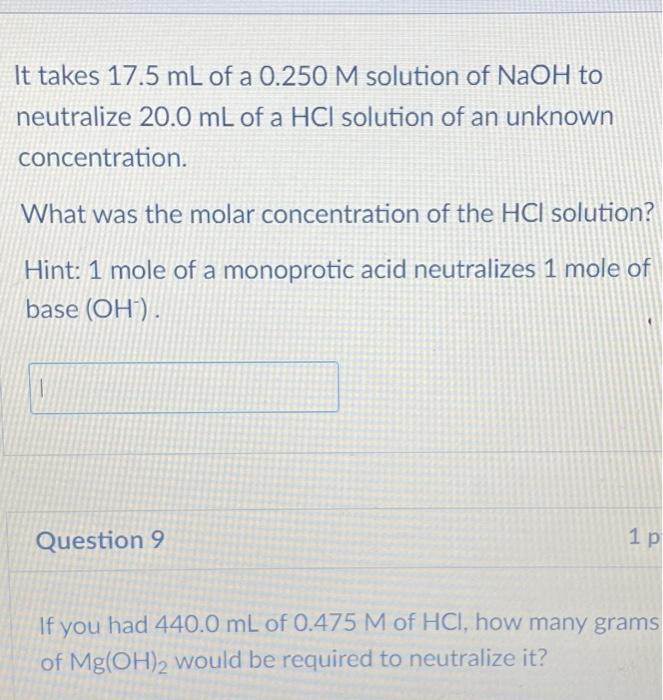
It takes of a solution of to neutralize of a solution of an unknown concentration. What was the molar concentration of the solution? Hint: 1 mole of a monoprotic acid neutralizes 1 mole of base . Question 9 If you had of of , how many grams of would be required to neutralize it?