(Solved): Leaching of a copper flotation concentrate The results of a laboratory investigation on the leaching ...
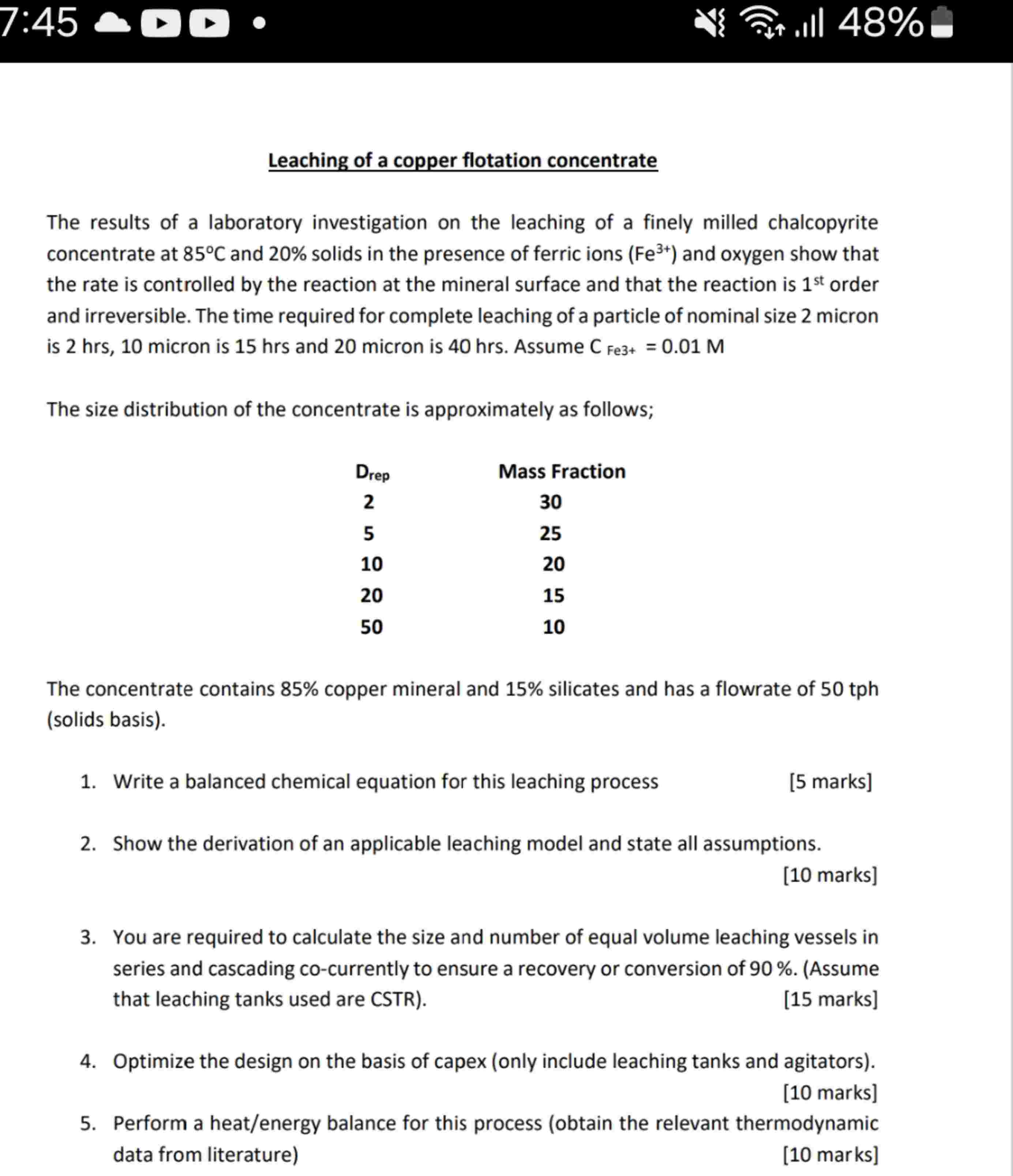
Leaching of a copper flotation concentrate The results of a laboratory investigation on the leaching of a finely milled chalcopyrite concentrate at \( 85^{\circ} \mathrm{C} \) and \( 20 \% \) solids in the presence of ferric ions ( \( \mathrm{Fe}^{3+} \) ) and oxygen show that the rate is controlled by the reaction at the mineral surface and that the reaction is \( 1^{\text {st }} \) order and irreversible. The time required for complete leaching of a particle of nominal size 2 micron is \( 2 \mathrm{hrs}, 10 \) micron is 15 hrs and 20 micron is 40 hrs . Assume \( \mathrm{C}_{\text {Fe3+ }}=0.01 \mathrm{M} \) The size distribution of the concentrate is approximately as follows; The concentrate contains 85\% copper mineral and 15\% silicates and has a flowrate of 50 tph (solids basis). 1. Write a balanced chemical equation for this leaching process 2. Show the derivation of an applicable leaching model and state all assumptions. [10 marks] 3. You are required to calculate the size and number of equal volume leaching vessels in series and cascading co-currently to ensure a recovery or conversion of \( 90 \% \). (Assume that leaching tanks used are CSTR). [15 marks] 4. Optimize the design on the basis of capex (only include leaching tanks and agitators). [10 marks] 5. Perform a heat/energy balance for this process (obtain the relevant thermodynamic data from literature) [10 marks]