Home /
Expert Answers /
Chemical Engineering /
methanol-is-produced-in-the-reaction-of-carbon-dioxide-and-hydrogen-co2-3h2ch3oh-h2-pa724
(Solved): Methanol is produced in the reaction of carbon dioxide and hydrogen: CO2+3H2CH3OH+H2 ...
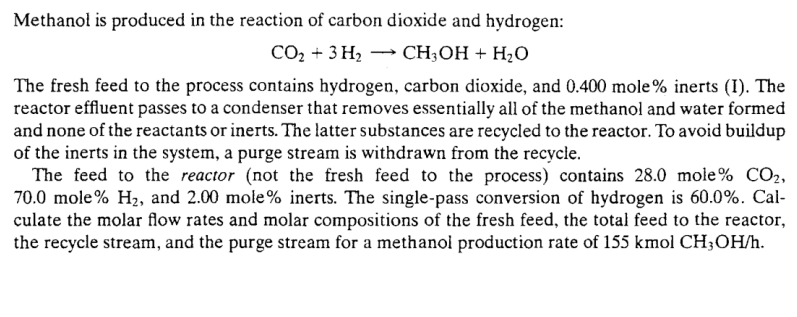
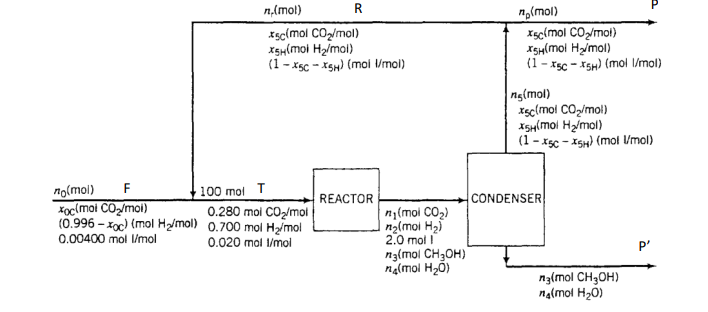
Methanol is produced in the reaction of carbon dioxide and hydrogen: The fresh feed to the process contains hydrogen, carbon dioxide, and inerts (I). The reactor effluent passes to a condenser that removes essentially all of the methanol and water formed and none of the reactants or inerts. The latter substances are recycled to the reactor. To avoid buildup of the inerts in the system, a purge stream is withdrawn from the recycle. The feed to the reactor (not the fresh feed to the process) contains 28.0 mole , 70.0 mole , and 2.00 mole inerts. The single-pass conversion of hydrogen is . Calculate the molar flow rates and molar compositions of the fresh feed, the total feed to the reactor, the recycle stream, and the purge stream for a methanol production rate of .