Home /
Expert Answers /
Chemistry /
nbsp-0-427-m-in-h2s-a-chegg-com-review-topics-references-use-the-references-to-access-importan-pa830
(Solved): 0.427 M In H2S A. Chegg.com [Review Topics (References) Use the References to access importan ...
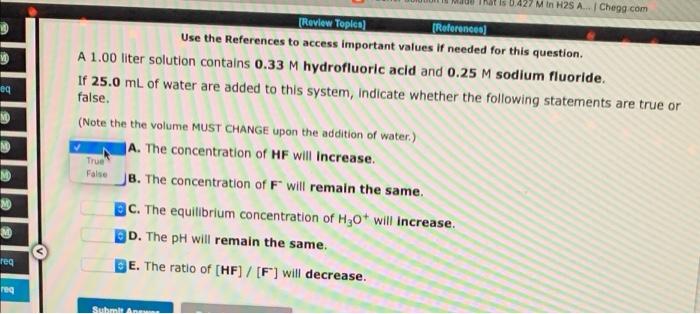

0.427 M In H2S A. Chegg.com [Review Topics (References) Use the References to access important values if needed for this question. A 1.00 liter solution contains 0.33 M hydrofluoric acid and 0.25 M sodium fluoride. If 25.0 mL of water are added to this system, indicate whether the following statements are true or false, (Note the the volume MUST CHANGE upon the addition of water.) A. The concentration of HF will increase. eg ME NO M True False M B. The concentration of F will remain the same. BC. The equilibrium concentration of H30+ will increase. D. The pH will remain the same. M rea E. The ratio of [HF] / [F] will decrease. re Sol A
A 1.00 liter solution contains 0.38 moles hydrocyanic acid and 0.29 moles sodium cyanide. If 0.15 moles of hydrobromic acid are added to this system, indicate whether the following statements are true or false. (Assume that the volume does not change upon the addition of hydrobromic acid.) A. The number of moles of HCN will decrease. B. The number of moles of CN will remain the same. C. The equilibrium concentration of H30+ will decrease. D. The pH will decrease. E. The ratio of [HCN] / (CN) will increase.
Expert Answer
A buffer is a solution which resist any change in pH on adding a small amount of acid or base . it is made up of weak acid and its conjugate salt or weak base and its conjugate salt. 1. A...false because on adding water, concentration decre