Home /
Expert Answers /
Calculus /
nbsp-how-would-i-do-this-boyle-39-s-law-states-that-if-the-temperature-of-a-gas-remains-constant-pa204
(Solved): how would I do this? Boyle's law states that if the temperature of a gas remains constant, ...
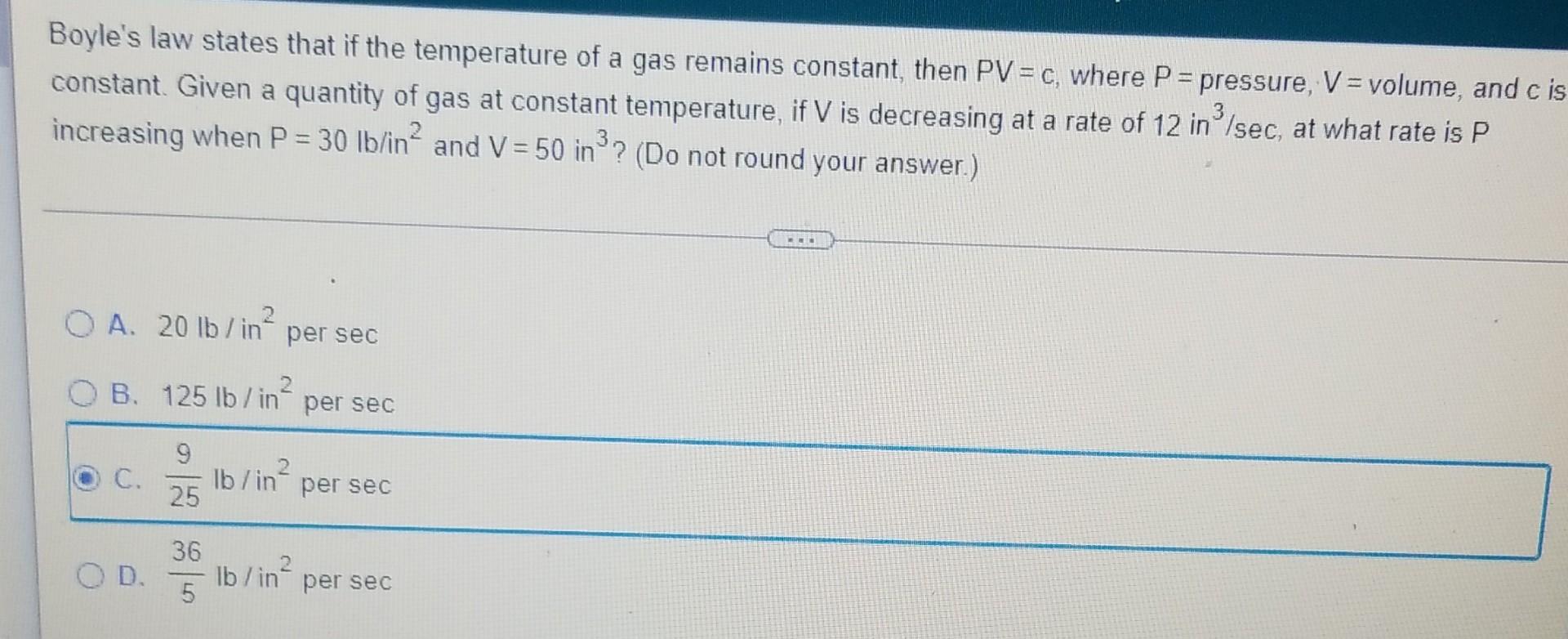
how would I do this?
Boyle's law states that if the temperature of a gas remains constant, then PV = c, where P = pressure, V = volume, and c is constant. Given a quantity of gas at constant temperature, if V is decreasing at a rate of 12 in /sec, at what rate is P increasing when P = 30 lb/in² and V = 50 in ³? (Do not round your answer.) OA. 20 lb/in² per sec B. 125 lb/in² per sec 9 25 C. OD. 36 5 lb/in per sec lb/in per sec