Home /
Expert Answers /
Chemistry /
nbsp-if-a-second-period-diatomic-molecule-has-16-electrons-in-its-valence-shell-total-of-2-pa497
(Solved): If a second-period diatomic molecule has 16 electrons in its valence shell (total of \( 2 ...
If a second-period diatomic molecule has 16 electrons in its valence shell (total of \( 2 \mathrm{~s} \) and \( 2 p \) electrons), what is the predicted bond order of the molecule? 0 1 \( 0.5 \) 2
What is the height of a column of liquid in a barometer if the density of the liquid is \( 0.99 \) \( \mathrm{g} / \mathrm{mL} \) and the local atmospheric pressure is \( 0.95 \) bar? \[ 9.79 \mathrm{~m} \] \( 0.0978 \mathrm{~m} \) \[ 9587 \mathrm{~m} \] Not enough information
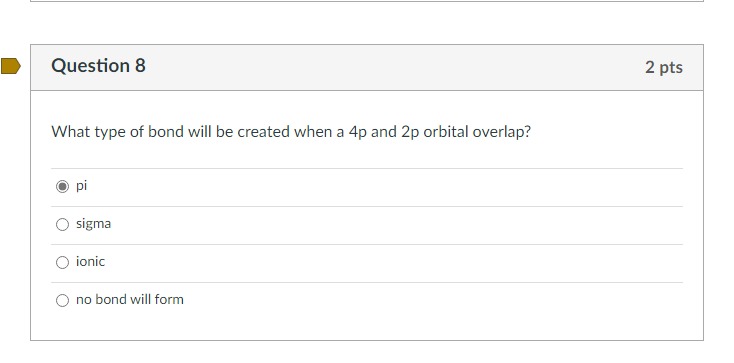
What type of bond will be created when a \( 4 p \) and \( 2 p \) orbital overlap? pi sigma ionic no bond will form