Home /
Expert Answers /
Chemistry /
nbsp-needed-asap-the-volume-of-a-sample-of-gas-is-650-mathrm-ml-at-0-00-circ-m-pa666
(Solved): needed asap The volume of a sample of gas is \( 650 \mathrm{~mL} \) at \( 0.00^{\circ} \m ...
needed asap
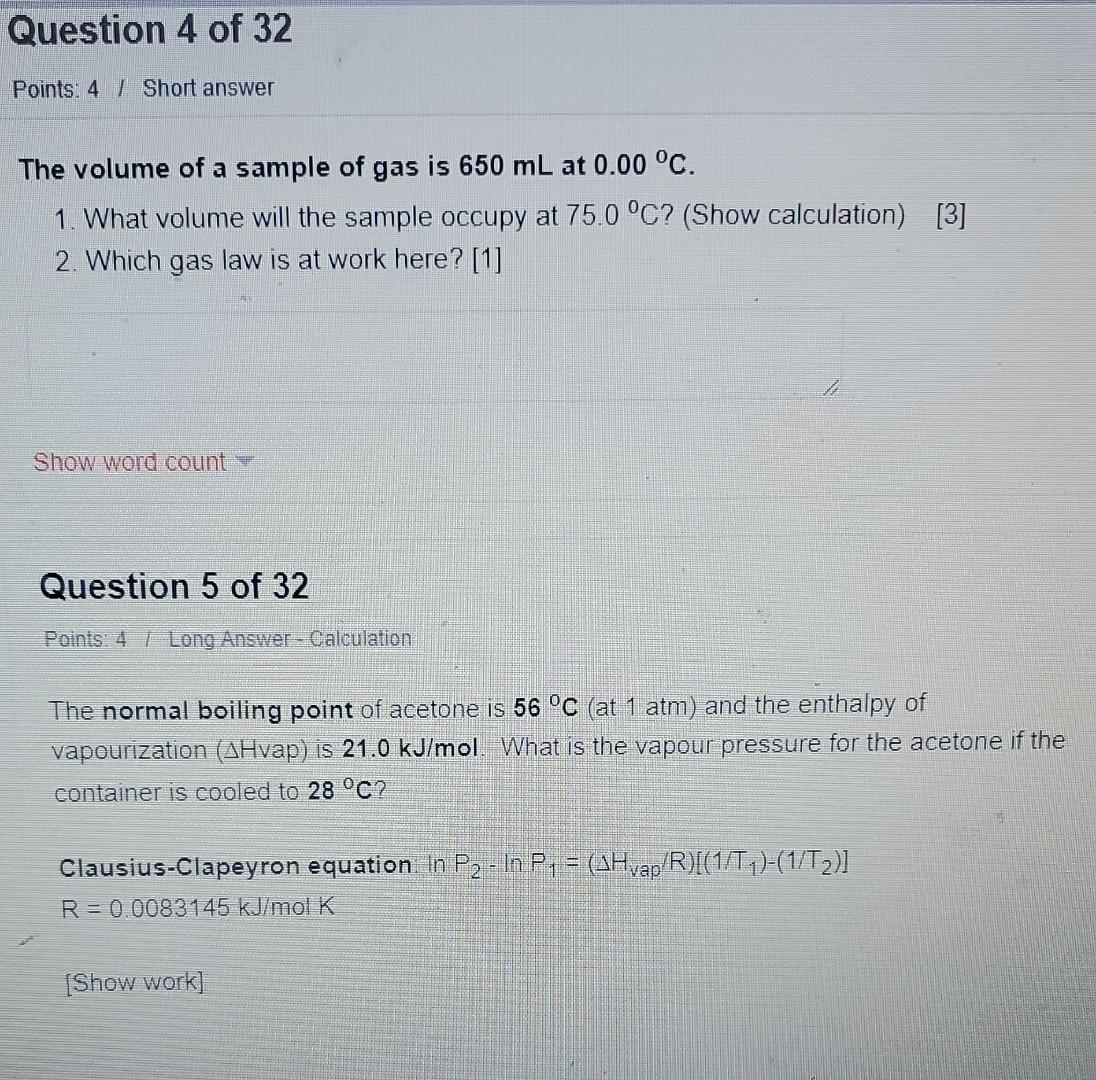
The volume of a sample of gas is \( 650 \mathrm{~mL} \) at \( 0.00^{\circ} \mathrm{C} \). 1. What volume will the sample occupy at \( 75.0{ }^{\circ} \mathrm{C} \) ? (Show calculation) \( [3] \) 2. Which gas law is at work here? [1] Show word count \( = \) Question 5 of 32 Points: 4 I Long Answer-Calculation The normal boiling point of acetone is \( 56{ }^{\circ} \mathrm{C} \) (at \( 1 \mathrm{~atm} \) ) and the enthalpy of vapourization ( \( \triangle \mathrm{Hvap} \) ) is \( 21.0 \mathrm{~kJ} / \mathrm{mol} \). What is the vapour pressure for the acetone if the container is cooled to \( 28^{\circ} \mathrm{C} \) ? Clausius-Clapeyron equation \( \ln P_{2}-\ln P_{1}=\left(\Delta H_{v a p}[R)\left[\left(1 / T_{1}\right)-\left(1 / T_{2}\right)\right]\right. \) \( R=0.0083145 \mathrm{~kJ} / \mathrm{mol} K \)
Expert Answer
Q4. 1) Here we have given that, V1 = 650 mL T1 = 0°C = 273k T2 = 75° C =