Home /
Expert Answers /
Chemistry /
need-help-lesson-2-calculating-ph-and-poh-1-complete-the-following-table-by-calculating-the-ph-and-pa534
(Solved): need help Lesson 2: Calculating pH and pOH 1. Complete the following table by calculating the pH and ...
need help
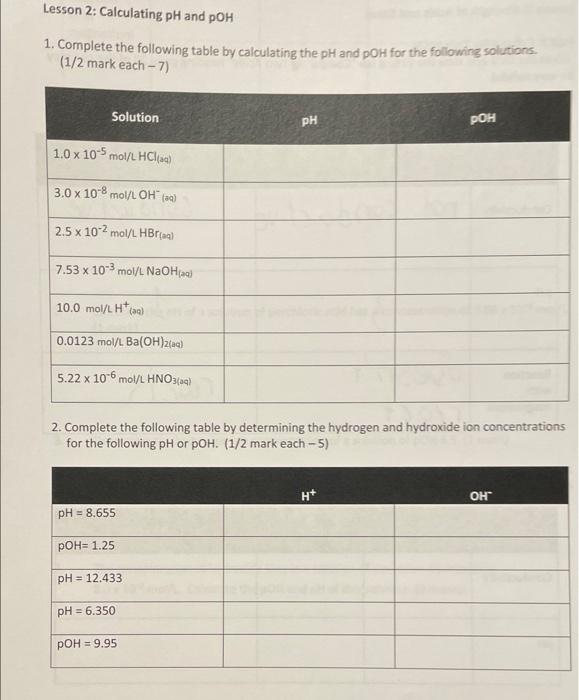

Lesson 2: Calculating pH and pOH 1. Complete the following table by calculating the pH and pOH for the following solutions. (1/2 mark each-7) Solution PH POH 1.0 x 105 mol/L HCl(aq) 3.0 x 108 mol/L OH(aq) 2.5 x 102 mol/L HBr(aq) 7.53 x 103 mol/L NaOH(aq) 10.0 mol/L H+ (aq) 0.0123 mol/L Ba(OH)2(aq) 5.22 x 10-6 mol/L HNO3(aq) 2. Complete the following table by determining the hydrogen and hydroxide ion concentrations for the following pH or pOH. (1/2 mark each - 5) H+ OH™ pH = 8.655 POH= 1.25 pH = 12.433 pH = 6.350 pOH = 9.95
Expert Answer
Solution: 1. pH + pOH = 14.00 pH = -log[H+] and pOH = -log[OH-] Solution pH pOH 1.0 x 10-5 mol/L HCl = 1.0 x 10-5 mol/L H+ -log(1.0 x 10-5) =